Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels
- PMID: 21811399
- PMCID: PMC3139627
- DOI: 10.1371/journal.pbio.1001107
Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels
Abstract
In the vertebrate retina, horizontal cells generate the inhibitory surround of bipolar cells, an essential step in contrast enhancement. For the last decades, the mechanism involved in this inhibitory synaptic pathway has been a major controversy in retinal research. One hypothesis suggests that connexin hemichannels mediate this negative feedback signal; another suggests that feedback is mediated by protons. Mutant zebrafish were generated that lack connexin 55.5 hemichannels in horizontal cells. Whole cell voltage clamp recordings were made from isolated horizontal cells and cones in flat mount retinas. Light-induced feedback from horizontal cells to cones was reduced in mutants. A reduction of feedback was also found when horizontal cells were pharmacologically hyperpolarized but was absent when they were pharmacologically depolarized. Hemichannel currents in isolated horizontal cells showed a similar behavior. The hyperpolarization-induced hemichannel current was strongly reduced in the mutants while the depolarization-induced hemichannel current was not. Intracellular recordings were made from horizontal cells. Consistent with impaired feedback in the mutant, spectral opponent responses in horizontal cells were diminished in these animals. A behavioral assay revealed a lower contrast-sensitivity, illustrating the role of the horizontal cell to cone feedback pathway in contrast enhancement. Model simulations showed that the observed modifications of feedback can be accounted for by an ephaptic mechanism. A model for feedback, in which the number of connexin hemichannels is reduced to about 40%, fully predicts the specific asymmetric modification of feedback. To our knowledge, this is the first successful genetic interference in the feedback pathway from horizontal cells to cones. It provides direct evidence for an unconventional role of connexin hemichannels in the inhibitory synapse between horizontal cells and cones. This is an important step in resolving a long-standing debate about the unusual form of (ephaptic) synaptic transmission between horizontal cells and cones in the vertebrate retina.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




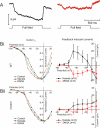


 in control condition, DNQX, and KA, produced by the ephaptic feedback model . Right: the DNQX- and KA-induced feedback currents. The value of
in control condition, DNQX, and KA, produced by the ephaptic feedback model . Right: the DNQX- and KA-induced feedback currents. The value of  was varied from 100% (A) to 10% (C) of the wild-type value . With reducing
was varied from 100% (A) to 10% (C) of the wild-type value . With reducing  , the DNQX-induced feedback currents decrease and finally reverse. The KA-induced feedback currents only decrease with decreasing
, the DNQX-induced feedback currents decrease and finally reverse. The KA-induced feedback currents only decrease with decreasing  .
.
Similar articles
-
Physiological and molecular characterization of connexin hemichannels in zebrafish retinal horizontal cells.J Neurophysiol. 2012 May;107(10):2624-32. doi: 10.1152/jn.01126.2011. Epub 2012 Feb 22. J Neurophysiol. 2012. PMID: 22357795 Free PMC article.
-
Hemichannel-mediated and pH-based feedback from horizontal cells to cones in the vertebrate retina.PLoS One. 2009 Jun 30;4(6):e6090. doi: 10.1371/journal.pone.0006090. PLoS One. 2009. PMID: 19564917 Free PMC article.
-
Localizing Proton-Mediated Inhibitory Feedback at the Retinal Horizontal Cell-Cone Synapse with Genetically-Encoded pH Probes.J Neurosci. 2019 Jan 23;39(4):651-662. doi: 10.1523/JNEUROSCI.1541-18.2018. Epub 2018 Nov 30. J Neurosci. 2019. PMID: 30504272 Free PMC article.
-
The involvement of glutamate-gated channels in negative feedback from horizontal cells to cones.Prog Brain Res. 2005;147:219-29. doi: 10.1016/S0079-6123(04)47017-4. Prog Brain Res. 2005. PMID: 15581709 Review.
-
Connexin hemichannel mediated ephaptic inhibition in the retina.Brain Res. 2012 Dec 3;1487:25-38. doi: 10.1016/j.brainres.2012.04.059. Epub 2012 Jul 13. Brain Res. 2012. PMID: 22796289 Review.
Cited by
-
Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina.Nat Neurosci. 2014 Feb;17(2):262-8. doi: 10.1038/nn.3627. Epub 2014 Jan 19. Nat Neurosci. 2014. PMID: 24441679 Free PMC article.
-
Role of connexin channels in the retinal light response of a diurnal rodent.Front Cell Neurosci. 2014 Aug 25;8:249. doi: 10.3389/fncel.2014.00249. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25202238 Free PMC article.
-
Diverse Cell Types, Circuits, and Mechanisms for Color Vision in the Vertebrate Retina.Physiol Rev. 2019 Jul 1;99(3):1527-1573. doi: 10.1152/physrev.00027.2018. Physiol Rev. 2019. PMID: 31140374 Free PMC article. Review.
-
An Ultrastructural and Immunohistochemical Analysis of the Outer Plexiform Layer of the Retina of the European Silver Eel (Anguilla anguilla L).PLoS One. 2016 Mar 31;11(3):e0152967. doi: 10.1371/journal.pone.0152967. eCollection 2016. PLoS One. 2016. PMID: 27032102 Free PMC article.
-
Molecular mechanisms underlying selective synapse formation of vertebrate retinal photoreceptor cells.Cell Mol Life Sci. 2020 Apr;77(7):1251-1266. doi: 10.1007/s00018-019-03324-w. Epub 2019 Oct 4. Cell Mol Life Sci. 2020. PMID: 31586239 Free PMC article. Review.
References
-
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. - PubMed
-
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

