Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review
- PMID: 21811403
- PMCID: PMC3139665
- DOI: 10.1371/journal.pmed.1001056
Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review
Abstract
Background: Improving the outcomes of HIV/AIDS treatment programs in resource-limited settings requires successful linkage of patients testing positive for HIV to pre-antiretroviral therapy (ART) care and retention in pre-ART care until ART initiation. We conducted a systematic review of pre-ART retention in care in Africa.
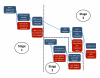
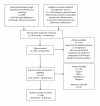
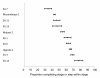
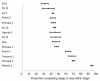
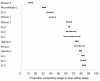
Methods and findings: We searched PubMed, ISI Web of Knowledge, conference abstracts, and reference lists for reports on the proportion of adult patients retained between any two points between testing positive for HIV and initiating ART in sub-Saharan African HIV/AIDS care programs. Results were categorized as Stage 1 (from HIV testing to receipt of CD4 count results or clinical staging), Stage 2 (from staging to ART eligibility), or Stage 3 (from ART eligibility to ART initiation). Medians (ranges) were reported for the proportions of patients retained in each stage. We identified 28 eligible studies. The median proportion retained in Stage 1 was 59% (35%-88%); Stage 2, 46% (31%-95%); and Stage 3, 68% (14%-84%). Most studies reported on only one stage; none followed a cohort of patients through all three stages. Enrollment criteria, terminology, end points, follow-up, and outcomes varied widely and were often poorly defined, making aggregation of results difficult. Synthesis of findings from multiple studies suggests that fewer than one-third of patients testing positive for HIV and not yet eligible for ART when diagnosed are retained continuously in care, though this estimate should be regarded with caution because of review limitations.
Conclusions: Studies of retention in pre-ART care report substantial loss of patients at every step, starting with patients who do not return for their initial CD4 count results and ending with those who do not initiate ART despite eligibility. Better health information systems that allow patients to be tracked between service delivery points are needed to properly evaluate pre-ART loss to care, and researchers should attempt to standardize the terminology, definitions, and time periods reported.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization, Joint United Nations Programme on HIV/AIDS, United Nations Children's Fund. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector; progress report 2010. Geneva: World Health Organization; 2010. Available: http://whqlibdoc.who.int/publications/2010/9789241500395_eng.pdf. Accessed 13 June 2011.
-
- Leisegang R, Cleary S, Hislop M, Davidse A, Regensberg L, et al. Early and late direct costs in a Southern African antiretroviral treatment programme: a retrospective cohort analysis. PLoS Med. 2009;6:e1000189. doi: 10.1371/journal.pmed.1000189. - DOI - PMC - PubMed
-
- Alcorn K. South Africa to launch mass HIV testing drive in April, to test 15 million in one year. 2010. Mar 25, AIDSMap News, NAM Publications. Available: http://www.aidsmap.com/South-Africa-to-launch-mass-HIV-testing-drive-in-.... Accessed 13 June 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

