Cable pili and the associated 22 kDa adhesin contribute to Burkholderia cenocepacia persistence in vivo
- PMID: 21811611
- PMCID: PMC3141045
- DOI: 10.1371/journal.pone.0022435
Cable pili and the associated 22 kDa adhesin contribute to Burkholderia cenocepacia persistence in vivo
Abstract
Background: Infection by Burkholderia cenocepacia in cystic fibrosis (CF) patients is associated with poor clinical prognosis. Previously, we demonstrated that one of the highly transmissible strains, BC7, expresses cable pili and the associated 22 kDa adhesin, both of which contribute to BC7 binding to airway epithelial cells. However, the contribution of these factors to induce inflammation and bacterial persistence in vivo is not known.
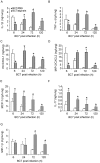
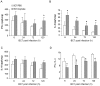
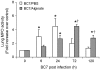
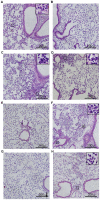
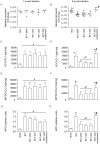
Methodology/principal findings: Wild-type BC7 stimulated higher IL-8 responses than the BC7 cbl and BC7 adhA mutants in both CF and normal bronchial epithelial cells. To determine the role of cable pili and the associated adhesin, we characterized a mouse model of B. cenocepacia, where BC7 are suspended in Pseudomonas aeruginosa alginate. C57BL/6 mice were infected intratracheally with wild-type BC7 suspended in either alginate or PBS and were monitored for lung bacterial load and inflammation. Mice infected with BC7 suspended in PBS completely cleared the bacteria by 3 days and resolved the inflammation. In contrast, mice infected with BC7 suspended in alginate showed persistence of bacteria and moderate lung inflammation up to 5 days post-infection. Using this model, mice infected with the BC7 cbl and BC7 adhA mutants showed lower bacterial loads and mild inflammation compared to mice infected with wild-type BC7. Complementation of the BC7 cblS mutation in trans restored the capacity of this strain to persist in vivo. Immunolocalization of bacteria revealed wild-type BC7 in both airway lumen and alveoli, while the BC7 cbl and BC7 adhA mutants were found mainly in airway lumen and peribronchiolar region.
Conclusions and significance: B. cenocepacia suspended in alginate can be used to determine the capacity of bacteria to persist and cause lung inflammation in normal mice. Both cable pili and adhesin contribute to BC7-stimulated IL-8 response in vitro, and BC7 persistence and resultant inflammation in vivo.
Conflict of interest statement
Figures
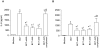



References
-
- Mahenthiralingam E, Baldwin A, Dowson CG. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol. 2008;104:1539–1551. - PubMed
-
- Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, et al. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol. 2008;58:1580–1590. - PubMed
-
- Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–156. - PubMed
-
- Isles A, Maclusky I, Corey M, Gold R, Prober C, et al. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

