Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression
- PMID: 21811631
- PMCID: PMC3141076
- DOI: 10.1371/journal.pone.0022544
Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression
Abstract
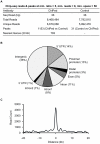
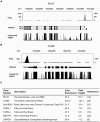
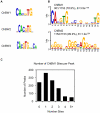
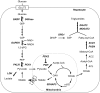
The carbohydrate response element binding protein (ChREBP), a basic helix-loop-helix/leucine zipper transcription factor, plays a critical role in the control of lipogenesis in the liver. To identify the direct targets of ChREBP on a genome-wide scale and provide more insight into the mechanism by which ChREBP regulates glucose-responsive gene expression, we performed chromatin immunoprecipitation-sequencing and gene expression analysis. We identified 1153 ChREBP binding sites and 783 target genes using the chromatin from HepG2, a human hepatocellular carcinoma cell line. A motif search revealed a refined consensus sequence (CABGTG-nnCnG-nGnSTG) to better represent critical elements of a functional ChREBP binding sequence. Gene ontology analysis shows that ChREBP target genes are particularly associated with lipid, fatty acid and steroid metabolism. In addition, other functional gene clusters related to transport, development and cell motility are significantly enriched. Gene set enrichment analysis reveals that ChREBP target genes are highly correlated with genes regulated by high glucose, providing a functional relevance to the genome-wide binding study. Furthermore, we have demonstrated that ChREBP may function as a transcriptional repressor as well as an activator.
Conflict of interest statement
Figures


References
-
- Cairo S, Merla G, Urbinati F, Ballabio A, Reymond A. WBSCR14, a gene mapping to the Williams–Beuren syndrome deleted region, is a new member of the Mlx transcription factor network. Hum Mol Genet. 2001;10:617–627. - PubMed
-
- Shih H-M, Liu Z, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem. 1995;270:21991–21997. - PubMed
-
- Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279:15662–15669. - PubMed

