Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway
- PMID: 21812036
- PMCID: PMC7771217
- DOI: 10.1002/glia.21219
Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway
Abstract
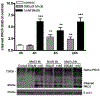
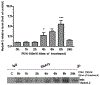
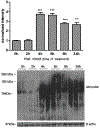
Manganese (Mn) is a trace element essential for normal human development and is required for the proper functioning of a variety of physiological processes. Chronic exposure to Mn can cause manganism, a neurodegenerative disorder resembling idiopathic Parkinson's disease (PD). Mn(II) neurotoxicity is characterized by astrocytic impairment both in the expression and activity of glutamine (Gln) transporters. Because protein kinase C (PKC) activation leads to the downregulation of a number of neurotransmitter transporters and Mn(II) increases PKC activity, we hypothesized that the PKC signaling pathway contributes to the Mn(II)-mediated disruption of Gln turnover. Our results have shown that Mn exposure increases the phosphorylation of both the PKCα and PKCδ isoforms. PKC activity was also shown to be increased in response to Mn(II) treatment. Corroborating our earlier observations, Mn(II) also caused a decrease in Gln uptake. This effect was blocked by PKC inhibitors. Notably, PKC activation caused a decrease in Gln uptake mediated by systems ASC and N, but had no effect on the activities of systems A and L. Exposure to α-phorbol 12-myristate 13-acetate significantly decreased SNAT3 (system N) and ASCT2 (system ASC) protein levels. Additionally, a co-immunoprecipitation study demonstrated the association of SNAT3 and ASCT2 with the PKCδ isoform, and Western blotting revealed the Mn(II)-mediated activation of PKCδ by proteolytic cleavage. PKC activation was also found to increase SNAT3 and ubiquitin ligase Nedd4-2 binding and to induce hyperubiquitination. Taken together, these findings demonstrate that the Mn(II)-induced dysregulation of Gln homeostasis in astrocytes involves PKCδ signaling accompanied by an increase in ubiquitin-mediated proteolysis.
Copyright © 2011 Wiley-Liss, Inc.
Figures
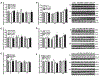
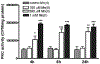
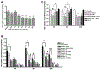
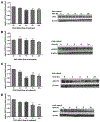

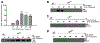
Similar articles
-
Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system.Glia. 2010 Dec;58(16):1905-12. doi: 10.1002/glia.21060. Glia. 2010. PMID: 20737472 Free PMC article.
-
Mechanism of Mn(II)-mediated dysregulation of glutamine-glutamate cycle: focus on glutamate turnover.J Neurochem. 2012 Aug;122(4):856-67. doi: 10.1111/j.1471-4159.2012.07835.x. Epub 2012 Jul 9. J Neurochem. 2012. PMID: 22708868 Free PMC article.
-
Manganese disrupts astrocyte glutamine transporter expression and function.J Neurochem. 2009 Aug;110(3):822-30. doi: 10.1111/j.1471-4159.2009.06172.x. Epub 2009 May 15. J Neurochem. 2009. PMID: 19457077 Free PMC article.
-
Impairment of glutamine/glutamate-γ-aminobutyric acid cycle in manganese toxicity in the central nervous system.Folia Neuropathol. 2014;52(4):377-82. doi: 10.5114/fn.2014.47838. Folia Neuropathol. 2014. PMID: 25574742 Review.
-
Role of Astrocytes in Manganese Neurotoxicity Revisited.Neurochem Res. 2019 Nov;44(11):2449-2459. doi: 10.1007/s11064-019-02881-7. Epub 2019 Sep 30. Neurochem Res. 2019. PMID: 31571097 Free PMC article. Review.
Cited by
-
The impact of manganese on neurotransmitter systems.J Trace Elem Med Biol. 2020 Sep;61:126554. doi: 10.1016/j.jtemb.2020.126554. Epub 2020 May 20. J Trace Elem Med Biol. 2020. PMID: 32480053 Free PMC article. Review.
-
Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions.Acta Neuropathol Commun. 2017 Nov 29;5(1):89. doi: 10.1186/s40478-017-0478-9. Acta Neuropathol Commun. 2017. PMID: 29187256 Free PMC article.
-
The Role of Oxidative Stress in Manganese Neurotoxicity: A Literature Review Focused on Contributions Made by Professor Michael Aschner.Biomolecules. 2023 Jul 28;13(8):1176. doi: 10.3390/biom13081176. Biomolecules. 2023. PMID: 37627240 Free PMC article. Review.
-
SMURF and NEDD4: sharp shooters monitor the gate keepers and ion traffic controllers of lead astray cell.J Membr Biol. 2011 Nov;244(1):1-8. doi: 10.1007/s00232-011-9394-2. Epub 2011 Sep 15. J Membr Biol. 2011. PMID: 21918841 Review.
-
PKC-Mediated Modulation of Astrocyte SNAT3 Glutamine Transporter Function at Synapses in Situ.Int J Mol Sci. 2018 Mar 21;19(4):924. doi: 10.3390/ijms19040924. Int J Mol Sci. 2018. PMID: 29561757 Free PMC article.
References
-
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. 1998. Acute regulation of norepinephrine transport. II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther 287:744–751. - PubMed
-
- Aschner M, Gannon M, Kimelberg HK. 1992. Manganese uptake and efflux in cultured rat astrocytes. J Neurochem 58:730–735. - PubMed
-
- Balkrishna S, Bröer A, Kingsland A, Bröer S. 2010. Rapid downregulation of the rat glutamine transporter SNAT3 by a caveolin-dependent trafficking mechanism in Xenopus laevis oocytes. Am J Physiol Cell Physiol 299:C1047–C1057. - PubMed
-
- Barbeau A 1984. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias). Neurotoxicology 5:13–35. - PubMed
-
- Battaini F 2001. Protein kinase C isoforms as therapeutic targets in nervous system disease states. Pharmacol Res 44:353–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

