Staphylococcus aureus sortase A contributes to the Trojan horse mechanism of immune defense evasion with its intrinsic resistance to Cys184 oxidation
- PMID: 21812416
- PMCID: PMC3604699
- DOI: 10.1021/bi200844h
Staphylococcus aureus sortase A contributes to the Trojan horse mechanism of immune defense evasion with its intrinsic resistance to Cys184 oxidation
Abstract
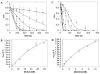
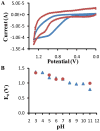
Staphylococcus aureus is a Gram-positive bacterial pathogen that causes serious infections which have become increasingly difficult to treat due to antimicrobial resistance and natural virulence strategies. Bacterial sortase enzymes are important virulence factors and good targets for future antibiotic development. It has recently been shown that sortase enzymes are integral to bacterial survival of phagocytosis, an underappreciated, but vital, step in S. aureus pathogenesis. Of note, the reaction mechanism of sortases relies on a solvent-accessible cysteine for transpeptidation. Because of the common strategy of oxidative damage employed by professional phagocytes to kill pathogens, it is possible that this cysteine may be oxidized inside the phagosome, thereby inhibiting the enzyme. This study addresses this apparent paradox by assessing the ability of physiological reactive oxygen species, hydrogen peroxide and hypochlorite, to inhibit sortase A (SrtA) from S. aureus. Surprisingly, we found that SrtA is highly resistant to oxidative inhibition, both in vitro and in vivo. The mechanism of resistance to oxidative damage is likely mediated by maintaining a high reduction potential of the catalytic cysteine residue, Cys184. This is due to the unusual active site utilized by S. aureus SrtA, which employs a reverse protonation mechanism for transpeptidation, resulting in a high pK(a) as well as reduction potential for Cys184. The results of this study suggest that S. aureus SrtA is able to withstand the extreme conditions encountered in the phagosome and maintain function, contributing to survival of phagocytotic killing.
Figures




Similar articles
-
Staphylococcus aureus sortase transpeptidase SrtA: insight into the kinetic mechanism and evidence for a reverse protonation catalytic mechanism.Biochemistry. 2005 Aug 23;44(33):11188-200. doi: 10.1021/bi050141j. Biochemistry. 2005. PMID: 16101303
-
Sortase from Staphylococcus aureus does not contain a thiolate-imidazolium ion pair in its active site.J Biol Chem. 2003 Sep 5;278(36):34061-5. doi: 10.1074/jbc.M305245200. Epub 2003 Jun 24. J Biol Chem. 2003. PMID: 12824164
-
Mutational analysis of active site residues in the Staphylococcus aureus transpeptidase SrtA.Biochemistry. 2007 Jun 19;46(24):7269-78. doi: 10.1021/bi700448e. Epub 2007 May 23. Biochemistry. 2007. PMID: 17518446
-
Sortase Transpeptidases: Structural Biology and Catalytic Mechanism.Adv Protein Chem Struct Biol. 2017;109:223-264. doi: 10.1016/bs.apcsb.2017.04.008. Epub 2017 Jun 5. Adv Protein Chem Struct Biol. 2017. PMID: 28683919 Free PMC article. Review.
-
Molecular features of the sortase enzyme family.FEBS J. 2015 Jun;282(11):2097-114. doi: 10.1111/febs.13288. Epub 2015 Apr 24. FEBS J. 2015. PMID: 25845800 Review.
Cited by
-
Kinetics and Optimization of the Lysine-Isopeptide Bond Forming Sortase Enzyme from Corynebacterium diphtheriae.Bioconjug Chem. 2020 Jun 17;31(6):1624-1634. doi: 10.1021/acs.bioconjchem.0c00163. Epub 2020 May 27. Bioconjug Chem. 2020. PMID: 32396336 Free PMC article.
-
Feedback-based, system-level properties of vertebrate-microbial interactions.PLoS One. 2013;8(2):e53984. doi: 10.1371/journal.pone.0053984. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437039 Free PMC article.
-
The "Lid" in the Streptococcus pneumoniae SrtC1 Sortase Adopts a Rigid Structure that Regulates Substrate Access to the Active Site.J Phys Chem B. 2016 Aug 25;120(33):8302-12. doi: 10.1021/acs.jpcb.6b01930. Epub 2016 May 5. J Phys Chem B. 2016. PMID: 27109553 Free PMC article.
-
Differential responses of osteoblasts and macrophages upon Staphylococcus aureus infection.BMC Microbiol. 2014 Jul 25;14:207. doi: 10.1186/s12866-014-0207-5. BMC Microbiol. 2014. PMID: 25059520 Free PMC article.
-
Toward an Alternative Therapeutic Approach for Skin Infections: Antagonistic Activity of Lactobacilli Against Antibiotic-Resistant Staphylococcus aureus and Pseudomonas aeruginosa.Probiotics Antimicrob Proteins. 2013 Sep;5(3):216-26. doi: 10.1007/s12602-013-9137-z. Probiotics Antimicrob Proteins. 2013. PMID: 26782990
References
-
- Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. Methicillin-resistant Staphylococcus aureus: the superbug. Int J Infect Dis. 2010;14(4):11. - PubMed
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. - PubMed
-
- Song JH. What's new on the antimicrobial horizon? Int J Antimicrob Agents. 2008;32(4):S207–213. - PubMed
-
- Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. - PubMed
-
- Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

