Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection
- PMID: 21812928
- PMCID: PMC3184377
- DOI: 10.1111/j.1600-6143.2011.03680.x
Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection
Abstract
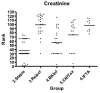
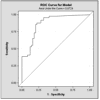
Renal transplant recipients require periodic surveillance for immune-based complications such as rejection and infection. Noninvasive monitoring methods are preferred, particularly for children, for whom invasive testing is problematic. We performed a cross-sectional analysis of adult and pediatric transplant recipients to determine whether a urine-based chemokine assay could noninvasively identify patients with rejection among other common clinical diagnoses. Urine was collected from 110 adults and 46 children with defined clinical conditions: healthy volunteers, stable renal transplant recipients, and recipients with clinical or subclinical acute rejection (AR) or BK infection (BKI), calcineurin inhibitor (CNI) toxicity or interstitial fibrosis (IFTA). Urine was analyzed using a solid-phase bead-array assay for the interferon gamma-induced chemokines CXCL9 and CXCL10. We found that urine CXCL9 and CXCL10 were markedly elevated in adults and children experiencing either AR or BKI (p = 0.0002), but not in stable allograft recipients or recipients with CNI toxicity or IFTA. The sensitivity and specificity of these chemokine assays exceeded that of serum creatinine. Neither chemokine distinguished between AR and BKI. These data show that urine chemokine monitoring identifies patients with renal allograft inflammation. This assay may be useful for noninvasively distinguishing those allograft recipients requiring more intensive surveillance from those with benign clinical courses.
©Copyright 2011 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures




References
-
- Davis ID, Oehlenschlager W, O’Riordan MA, Avner ED. Pediatric renal biopsy: should this procedure be performed in an outpatient setting? Pediatr Nephrol. 1998;12(2):96–100. - PubMed
-
- Pape L, Offner G, Ehrich JH, de Boer J, Persijn GG. Renal allograft function in matched pediatric and adult recipient pairs of the same donor. Transplantation. 2004;77(8):1191–1194. - PubMed
-
- Provoost AP, Wolff ED, de Keijzer MH, Molenaar JC. Influence of the recipient’s size upon renal function following kidney transplantation. An experimental and clinical investigation. J Pediatr Surg. 1984;19(1):63–67. - PubMed
-
- Hymes LC, Greenbaum L, Amaral SG, Warshaw BL. Surveillance renal transplant biopsies and subclinical rejection at three months post-transplant in pediatric patients. Pediatr Transplant. 2007;11(5):536–539. - PubMed
-
- Mengel M, Chapman JR, Cosio FG, Cavaillé-Coll MW, Haller H, Halloran PF, et al. Protocol biopsies in renal transplantation: insights into patient management and pathogenesis. Am J Transplant. 2007;7(3):512–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

