Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: mechanistic insights
- PMID: 21813271
- PMCID: PMC3209497
- DOI: 10.1016/j.jnutbio.2011.03.012
Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: mechanistic insights
Abstract
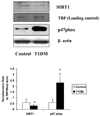
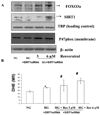
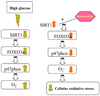
Several lines of evidence support a role for oxidative stress in diabetic complications. Diabetic patients have increased O(2)(-) production in monocytes. Loss of SIRT1 activity may be associated with metabolic diseases such as diabetes. Several studies have shown that SIRT1 can regulate mammalian FOXO transcription factors through direct binding and/or deacetylation. However, interactions between SIRT1 and FOXO under diabetic conditions are unclear. The phytochemical resveratrol has recently gained attention for its protection against metabolic disease. Resveratrol has been shown to increase mitochondrial function by activating SIRT1. In this study, we tested the protective effect of resveratrol on cellular oxidative stress through the SIRT1-FOXO pathway under high-glucose conditions. Human monocytic (THP-1) cells were cultured in the presence of mannitol (osmolar control) or normoglycemic (NG, 5.5 mmol/l glucose) or hyperglycemic (HG, 25 mmol/l glucose) conditions in absence or presence of resveratrol (3 and 6 μmol/l) for 48 h. We first examined SIRT1 activity and oxidative stress in monocytes of Type 1 diabetes mellitus (T1DM) patients compared with healthy controls. In T1DM patients, monocytic SIRT1 expression was significantly decreased and p47phox expression was increased compared with controls. Under HG in vitro, SIRT1 and FOXO3a were significantly decreased compared with NG, and this was reversed by resveratrol treatment, concomitant with reduction in HG-induced superoxide production and p47phox. Under HG, SIRT1 small interfering RNA (siRNA) inhibited FOXO3a, and there was no beneficial effect of resveratrol in siRNA-treated HG-induced cells. Thus, resveratrol decreases HG-induced superoxide production via up-regulation of SIRT1, induction of FOXO3a and inhibition of p47phox in monocytes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;6:1331–1336. - PubMed
-
- Jain SK, Kannan K, Lim G, Matthew-Greer J, McVie R, Bocchini JA. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–2143. - PubMed
-
- Shanmugam N, Reddy MA, Guha M, Natarajan R. High-glucose–induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–1264. - PubMed
-
- Ceolotto G, Gallo A, Miola M, Sartori M, Trevisan R, Prato SD, Semplicini A, Avogaro A. Protein kinase C activity is acutely regulated by plasma glucose concentration in human monocytes in vivo. Diabetes. 1999;48:1316–1322. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

