TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway
- PMID: 21813451
- PMCID: PMC3175780
- DOI: 10.1182/blood-2011-04-348839
TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway
Abstract
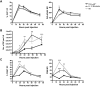
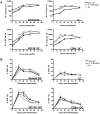
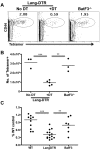
Conjugation of TLR agonists to protein or peptide antigens has been demonstrated in many studies to be an effective vaccine formula in inducing cellular immunity. However, the molecular and cellular mediators involved in TLR-induced immune responses have not been carefully examined. In this study, we identify Type I IFN and IL-12 as critical mediators of cross-priming induced by a TLR7 agonist-antigen conjugate. We demonstrate that TLR7-driven cross-priming requires both Type I IFN and IL-12. Signaling through the IFN-αβR was required for the timely recruitment and accumulation of activated dendritic cells in the draining lymph nodes. Although IL-12 was indispensable during cross-priming, it did not regulate DC function. Therefore, the codependency for these 2 cytokines during TLR7-induced cross-priming is the result of their divergent effects on different cell-types. Furthermore, although dermal and CD8α(+) DCs were able to cross-prime CD8(+) T cells, Langerhans cells were unexpectedly found to potently cross-present antigen and support CD8(+) T-cell expansion, both in vitro and in vivo. Collectively, the data show that a TLR7 agonist-antigen conjugate elicits CD8(+) T-cell responses by the coordinated recruitment and activation of both tissue-derived and lymphoid organ-resident DC subsets through a Type I IFN and IL-12 codependent mechanism.
Figures

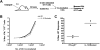


Similar articles
-
Influenza A infection enhances cross-priming of CD8+ T cells to cell-associated antigens in a TLR7- and type I IFN-dependent fashion.J Immunol. 2010 Nov 15;185(10):6013-22. doi: 10.4049/jimmunol.1002129. Epub 2010 Oct 18. J Immunol. 2010. PMID: 20956347
-
Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis.Hepatology. 2014 Jul;60(1):237-49. doi: 10.1002/hep.26981. Epub 2014 May 27. Hepatology. 2014. PMID: 24375615 Free PMC article.
-
Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets.J Clin Invest. 2011 May;121(5):1782-96. doi: 10.1172/JCI45416. Epub 2011 Apr 11. J Clin Invest. 2011. PMID: 21540549 Free PMC article.
-
TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8α+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface.J Immunol. 2013 Feb 1;190(3):948-60. doi: 10.4049/jimmunol.1102725. Epub 2013 Jan 2. J Immunol. 2013. PMID: 23284054
-
Type I interferon as a stimulus for cross-priming.Cytokine Growth Factor Rev. 2008 Feb;19(1):33-40. doi: 10.1016/j.cytogfr.2007.10.007. Cytokine Growth Factor Rev. 2008. PMID: 18068417 Review.
Cited by
-
Critical role of TLR7 signaling in the priming of cross-protective cytotoxic T lymphocyte responses by a whole inactivated influenza virus vaccine.PLoS One. 2013 May 2;8(5):e63163. doi: 10.1371/journal.pone.0063163. Print 2013. PLoS One. 2013. PMID: 23658804 Free PMC article.
-
The immunobiology of toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants.Shock. 2013 Dec;40(6):451-62. doi: 10.1097/SHK.0000000000000042. Shock. 2013. PMID: 23989337 Free PMC article. Review.
-
Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants.ACS Biomater Sci Eng. 2017 Feb 13;3(2):179-194. doi: 10.1021/acsbiomaterials.6b00408. Epub 2016 Nov 9. ACS Biomater Sci Eng. 2017. PMID: 29046894 Free PMC article.
-
Retrovirus-Based Virus-Like Particle Immunogenicity and Its Modulation by Toll-Like Receptor Activation.J Virol. 2017 Oct 13;91(21):e01230-17. doi: 10.1128/JVI.01230-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28794025 Free PMC article.
-
TRIF is required for TLR4 mediated adjuvant effects on T cell clonal expansion.PLoS One. 2013;8(2):e56855. doi: 10.1371/journal.pone.0056855. Epub 2013 Feb 15. PLoS One. 2013. PMID: 23457630 Free PMC article.
References
-
- Takeda K, Kaisho T., Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. - PubMed
-
- Doxsee C, Riter T, Reiter M, Gibson S, Vasilakos J, Kedl R. The immune response modifier and toll-like receptor 7 agonist S-27609 selectively induces IL-12 and TNF-a production in CD11c+ CD11b+ CD8- dendritic cells. J Immunol. 2003;171:1156–1163. - PubMed
-
- Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4(10):1009–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

