Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis
- PMID: 21813473
- PMCID: PMC3322588
- DOI: 10.1136/gutjnl-2011-300012
Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis
Abstract
Background: Inflammatory bowel diseases, encompassing Crohn's disease and ulcerative colitis, are characterised by persistent leucocyte tissue infiltration leading to perpetuation of an inappropriate inflammatory cascade. The neuronal guidance molecule netrin-1 has recently been implicated in the orchestration of leucocyte trafficking during acute inflammation. We therefore hypothesised that netrin-1 could modulate leucocyte infiltration and disease activity in a model of inflammatory bowel disease.
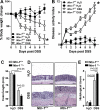
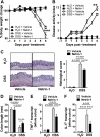
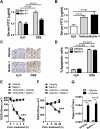
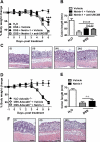
Design: DSS-colitis was performed in mice with partial genetic netrin-1 deficiency (Ntn-1(+/-) mice) or wild-type mice treated with exogenous netrin-1 via osmotic pump to examine the role of endogenous and therapeutically administered netrin-1. These studies were supported by in vitro models of transepithelial migration and intestinal epithelial barrier function.
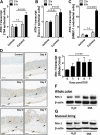
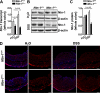
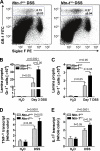
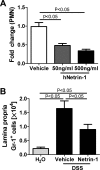
Results: Consistent with our hypothesis, we observed induction of netrin-1 during intestinal inflammation in vitro or in mice exposed to experimental colitis. Moreover, mice with partial netrin-1 deficiency demonstrated an exacerbated course of DSS-colitis compared to littermate controls, with enhanced weight loss and colonic shortening. Conversely, mice treated with exogenous mouse netrin-1 experienced attenuated disease severity. Importantly, permeability studies and quantitative assessment of apoptosis reveal that netrin-1 signalling events do not alter mucosal permeability or intestinal epithelial cell apoptosis. In vivo studies of leucocyte transmigration demonstrate suppression of neutrophil trafficking as a key function mediated by endogenous or exogenously administered netrin-1. Finally, genetic studies implicate the A2B adenosine receptor in netrin-1-mediated protection during DSS-colitis.
Conclusions: The present study identifies a previously unrecognised role for netrin-1 in attenuating experimental colitis through limitation of neutrophil trafficking.
Conflict of interest statement
Figures
References
-
- Serafini T, Kennedy TE, Galko MJ, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 1994;78:409–24 - PubMed
-
- Rosenberger P, Schwab JM, Mirakaj V, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 2009;10:195–202 - PubMed
-
- Yebra M, Montgomery AM, Diaferia GR, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell 2003;5:695–707 - PubMed
-
- Strizzi L, Bianco C, Raafat A, et al. Netrin-1 regulates invasion and migration of mouse mammary epithelial cells overexpressing Cripto-1 in vitro and in vivo. J Cell Sci 2005;118:4633–43 - PubMed
-
- Wang W, Reeves WB, Ramesh G. Netrin-1 increases proliferation and migration of renal proximal tubular epithelial cells via the UNC5B receptor. Am J Physiol Renal Physiol 2009;296:F723–9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
