Genital warts and cost of care in England
- PMID: 21813567
- PMCID: PMC3253068
- DOI: 10.1136/sti.2010.048421
Genital warts and cost of care in England
Abstract
Objectives: To estimate the total number of cases of, and cost of care for, genital warts (GWs) in England, to inform economic evaluations of human papillomavirus vaccination.
Methods: The number of GW cases seen in general practices (GPs) and in genitourinary medicine (GUM) clinics was estimated using the General Practice Research Database and the GUM Clinic Activity Dataset. The overlap in care of cases in the two settings was estimated. The calculated costs of care in GP and hospitals were added to the costs of care in GUM clinics (estimated elsewhere) to estimate the cost of care for GWs in England.
Results: In England, in 2008, GP and GUM saw 80,531 new (157/100,000 population) and 68,259 recurrent (133/100,000 population) episodes, giving a total of 148,790 episodes of care of GWs (289/100,000 population). Seventy-three per cent of cases were seen only in GUM clinics, 22% were seen by a GP before being referred to GUM, and 5% by GPs only. Hospital care was given in 1.3% of cases and contributed 8% of the costs. The average cost of care per episode was £113, and the estimated annual cost of care in England was £16.8 million.
Conclusions: This study provides a fairly comprehensive measure of GW frequency and care in England. GWs exert a considerable impact on health services, a large proportion of which could be prevented through immunisation using the quadrivalent human papillomavirus vaccine.
Conflict of interest statement
Figures

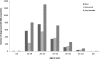
References
-
- Health Protection Agency STI Annual Data Tables. 2009. http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1203348026613 (accessed 25 Aug 2010).
-
- British Association for Sexual Health and HIV United Kingdom National Guideline on the Management of Ano-genital Warts. 2007. http://www.bashh.org/documents/86/86.pdf (accessed 24 Aug 2010).
-
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010;28:6247–55 - PubMed
-
- The FUTURE II Group Effect of prophylactic human papillomavirus L1 virus-like particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007;369:1861–8 - PubMed
-
- Dillner J, Kjaer SK, Wheeler CM, et al. ; The FUTURE I/II Study Group Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 2010;341:c3493. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
