Medication adherence assessment in a clinical trial with centralized follow-up and direct-to-patient drug shipments
- PMID: 21813583
- PMCID: PMC6178218
- DOI: 10.1177/1740774511410331
Medication adherence assessment in a clinical trial with centralized follow-up and direct-to-patient drug shipments
Abstract
Background: Assessment of adherence to study medications is a common challenge in clinical research. Counting unused study medication is the predominant method by which adherence is assessed in outpatient clinical trials but it has limitations that include questionable validity and burdens on research personnel.
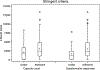
Purpose: To compare capsule counts, patient questionnaire responses, and plasma drug levels as methods of determining adherence in a clinical trial that had 2056 participants and used centralized drug distribution and patient follow-up.
Methods: Capsule counts from study medication bottles returned by participants and responses to questions regarding adherence during quarterly telephone interviews were averaged and compared. Both measures were compared to plasma drug levels obtained at the 3-month study visit of patients in the treatment group. Counts and questionnaire responses were converted to adherence rates (doses taken divided by days elapsed) and were categorized by stringent (≥85.7%) and liberal (≥71.4%) definitions. We calculated the prevalence-adjusted bias-adjusted kappa to assess agreement between the two measures.
Results: Using a pre-paid mailer, participants returned 76.0% of study medication bottles to the central pharmacy. Both capsule counts and questionnaire responses were available for 65.8% of participants and were used to assess adherence. Capsule counts identified more patients who were under-adherent (18.8% by the stringent definition and 7.5% by the liberal definition) than self-reports did (10.4% by the stringent definition and 2.1% by the liberal definition). The prevalence-adjusted bias-adjusted kappa was 0.58 (stringent) and 0.83 (liberal), indicating fair and very good agreement, respectively. Both measures were also in agreement with plasma drug levels determined at the 3-month visit (capsule counts: p = 0.005 for the stringent and p = 0.003 for the liberal definition; questionnaire: p = 0.002 for both adherence definitions).
Limitations: Inconsistent bottle returns and incomplete notations of medication start and stop dates resulted in missing data but exploratory missing data analyses showed no reason to believe that the missing data resulted in systematic bias.
Conclusions: Depending upon the definition of adherence, there was fair to very good agreement between questionnaire results and capsule counts among returned study bottles, confirmed by plasma drug levels. We conclude that a self-report of medication adherence is potentially comparable to capsule counts as a method of assessing adherence in a clinical trial, if a relatively low adherence threshold is acceptable, but adherence should be confirmed by other measures if a high adherence threshold is required.
Figures

References
-
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353:487–97. - PubMed
-
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 1999; 21: 1074–90. - PubMed
-
- Jayaraman S, Rieder MJ, Matsui DM. Compliance assessment in drug trials: has there been improvement in two decades?. Can J Clin Pharmacol 2005; 12:e251–53. - PubMed
-
- Friedman LM, Furberg CD, DeMets DL. Participant adherence Fundamentals of Clinical Trials (3rd edn, Chapter 13). Springer-Verlag, New York, 1998.
-
- Matsui D, Hermann C, Klein J, et al. Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. J Clin Pharmacol 1994; 34: 944–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

