Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone
- PMID: 21813597
- PMCID: PMC3187515
- DOI: 10.1128/JVI.00849-11
Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone
Abstract
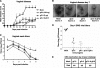
Attempts to develop a vaccine to prevent genital herpes simplex virus 2 (HSV-2) disease have been only marginally successful, suggesting that novel strategies are needed. Immunization with HSV-2 glycoprotein C (gC-2) and gD-2 was evaluated in mice and guinea pigs to determine whether adding gC-2 to a gD-2 subunit vaccine would improve protection by producing antibodies that block gC-2 immune evasion from complement. Antibodies produced by gC-2 immunization blocked the interaction between gC-2 and complement C3b, and passive transfer of gC-2 antibody protected complement-intact mice but not C3 knockout mice against HSV-2 challenge, indicating that gC-2 antibody is effective, at least in part, because it prevents HSV-2 evasion from complement. Immunization with gC-2 also produced neutralizing antibodies that were active in the absence of complement; however, the neutralizing titers were higher when complement was present, with the highest titers in animals immunized with both antigens. Animals immunized with the gC-2-plus-gD-2 combination had robust CD4+ T-cell responses to each immunogen. Multiple disease parameters were evaluated in mice and guinea pigs immunized with gC-2 alone, gD-2 alone, or both antigens. In general, gD-2 outperformed gC-2; however, the gC-2-plus-gD-2 combination outperformed gD-2 alone, particularly in protecting dorsal root ganglia in mice and reducing recurrent vaginal shedding of HSV-2 DNA in guinea pigs. Therefore, the gC-2 subunit antigen enhances a gD-2 subunit vaccine by stimulating a CD4+ T-cell response, by producing neutralizing antibodies that are effective in the absence and presence of complement, and by blocking immune evasion domains that inhibit complement activation.
Figures










References
-
- Bryson Y. J., et al. 1983. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N. Engl. J. Med. 308:916–921 - PubMed
-
- Byars N. E., et al. 1994. Vaccinating guinea pigs with recombinant glycoprotein D of herpes simplex virus in an efficacious adjuvant formulation elicits protection against vaginal infection. Vaccine 12:200–209 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

