A Kaposi's sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice
- PMID: 21813606
- PMCID: PMC3196388
- DOI: 10.1128/JVI.05558-11
A Kaposi's sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice
Abstract
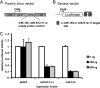
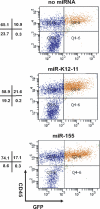
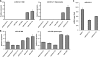
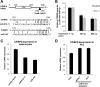
MicroRNAs (miRNAs) are small noncoding RNA molecules that function as posttranscriptional regulators of gene expression. Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), a B-cell-tropic virus associated with KS and B-cell lymphomas, encodes 12 miRNA genes that are highly expressed in these tumor cells. One viral miRNA, miR-K12-11, shares 100% seed sequence homology with hsa-miR-155, an oncogenic human miRNA that functions as a key regulator of hematopoiesis and B-cell differentiation. So far, in vitro studies have shown that both miRNAs can regulate a common set of cellular target genes, suggesting that miR-K12-11 may mimic miR-155 function. To comparatively study miR-K12-11 and miR-155 function in vivo, we used a foamy virus vector to express the miRNAs in human hematopoietic progenitors and performed immune reconstitutions in NOD/LtSz-scid IL2Rγ(null) mice. We found that ectopic expression of miR-K12-11 or miR-155 leads to a significant expansion of the CD19(+) B-cell population in the spleen. Subsequent quantitative PCR analyses of these splenic B cells revealed that C/EBPβ, a transcriptional regulator of interleukin-6 that is linked to B-cell lymphoproliferative disorders, is downregulated when either miR-K12-11 or miR-155 is ectopically expressed. In addition, inhibition of miR-K12-11 function using antagomirs in KSHV-infected human primary effusion lymphoma B cells resulted in derepression of C/EBPβ transcript levels. This in vivo study validates miR-K12-11 as a functional ortholog of miR-155 in the context of hematopoiesis and suggests a novel mechanism by which KSHV miR-K12-11 induces splenic B-cell expansion and potentially KSHV-associated lymphomagenesis by targeting C/EBPβ.
Figures

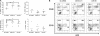
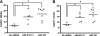

References
-
- Asou H., et al. 1998. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 91:2475–2481 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

