Low-pH-dependent changes in the conformation and oligomeric state of the prefusion form of herpes simplex virus glycoprotein B are separable from fusion activity
- PMID: 21813610
- PMCID: PMC3196434
- DOI: 10.1128/JVI.05291-11
Low-pH-dependent changes in the conformation and oligomeric state of the prefusion form of herpes simplex virus glycoprotein B are separable from fusion activity
Abstract
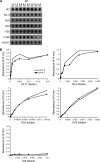
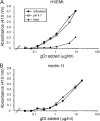
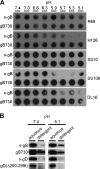
The cellular requirements for activation of herpesvirus fusion and entry remain poorly understood. Low pH triggers change in the antigenic reactivity of the prefusion form of the herpes simplex virus (HSV) fusion protein gB in virions, both in vitro and during viral entry via endocytosis (S. Dollery et al., J. Virol. 84:3759-3766, 2010). However, the mechanism and magnitude of gB conformational change are not clear. Here we show that the conformation and oligomeric state of gB with mutations in the bipartite fusion loops were similarly altered despite the fusion-inactivating mutations. Together with previous studies, this suggests that fusion loop mutants undergo conformational changes but are defective for fusion because they fail to make productive contact with the outer leaflet of the host target membrane. A direct, reversible effect of low pH on the structure of gB was detected by fluorescence spectroscopy. A soluble form of gB containing cytoplasmic tail sequences (s-gB) was triggered by mildly acidic pH to undergo changes in tryptophan fluorescence emission, hydrophobicity, antigenic conformation, and oligomeric structure and thus resembled the prefusion form of gB in the virion. In contrast, soluble gB730, for which the postfusion crystal structure is known, was only marginally affected by pH using these measures. The results underscore the importance of using a prefusion form of gB to assess the activation and extent of conformation change. Further, acidic pH had little to no effect on the conformation or hydrophobicity of gD or on gD's ability to bind nectin-1 or HVEM receptors. Our results support a model in which endosomal low pH serves as a cellular trigger of fusion by activating conformational changes in the fusion protein gB.
Figures
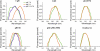

References
-
- Arii J., et al. 2010. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 467:859–862 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

