Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent
- PMID: 21813611
- PMCID: PMC3187518
- DOI: 10.1128/JVI.05408-11
Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent
Abstract
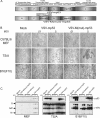
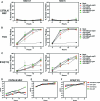
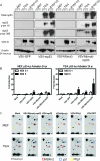
Vesicular stomatitis virus (VSV), a negative-strand RNA rhabdovirus, preferentially replicates in and eradicates transformed versus nontransformed cells and is thus being considered for use as a potential anticancer treatment. The genetic malleability of VSV also affords an opportunity to develop more potent agents that exhibit increased therapeutic activity. The tumor suppressor p53 has been shown to exert potent antitumor properties, which may in part involve stimulating host innate immune responses to malignancies. To evaluate whether VSV expressing p53 exhibited enhanced oncolytic action, the murine p53 (mp53) gene was incorporated into recombinant VSVs with or without a functional viral M gene-encoded protein that could either block (VSV-mp53) or enable [VSV-M(mut)-mp53] host mRNA export following infection of susceptible cells. Our results indicated that VSV-mp53 and VSV-M(mut)-mp53 expressed high levels of functional p53 and retained the ability to lyse transformed versus normal cells. In addition, we observed that VSV-ΔM-mp53 was extremely attenuated in vivo due to p53 activating innate immune genes, such as type I interferon (IFN). Significantly, immunocompetent animals with metastatic mammary adenocarcinoma exhibited increased survival following treatment with a single inoculation of VSV-ΔM-mp53, the mechanisms of which involved enhanced CD49b+ NK and tumor-specific CD8+ T cell responses. Our data indicate that VSV incorporating p53 could provide a safe, effective strategy for the design of VSV oncolytic therapeutics and VSV-based vaccines.
Figures



References
-
- Adkins B., Bu Y., Cepero E., Perez R. 2000. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J. Immunol. 164:2347–2353 - PubMed
-
- Angelo L. S., Talpaz M., Kurzrock R. 2002. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 62:932–940 - PubMed
-
- Arai K., Liu Z. X., Lane T., Dennert G. 2002. IP-10 and Mig facilitate accumulation of T cells in the virus-infected liver. Cell Immunol. 219:48–56 - PubMed
-
- Balachandran S., Barber G. N. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51–65 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

