Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2
- PMID: 21813614
- PMCID: PMC3196435
- DOI: 10.1128/JVI.00545-11
Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2
Abstract
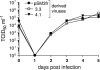
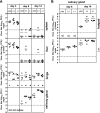
Murine cytomegalovirus (MCMV) Smith strain has been cloned as a bacterial artificial chromosome (BAC) named pSM3fr and used for analysis of virus gene functions in vitro and in vivo. When sequencing the complete BAC genome, we identified a frameshift mutation within the open reading frame (ORF) encoding MCMV chemokine homologue MCK-2. This mutation would result in a truncated MCK-2 protein. When mice were infected with pSM3fr-derived virus, we observed reduced virus production in salivary glands, which could be reverted by repair of the frameshift mutation. When looking for the source of the mutation, we consistently found that virus stocks of cell culture-passaged MCMV Smith strain are mixtures of viruses with or without the MCK-2 mutation. We conclude that the MCK-2 mutation in the pSM3fr BAC is the result of clonal selection during the BAC cloning procedure.
Figures


References
-
- Ahasan M. M., Sweet C. 2007. Murine cytomegalovirus open reading frame m29.1 augments virus replication both in vitro and in vivo. J. Gen. Virol. 88:2941–2951 - PubMed
-
- Brune W., Menard C., Heesemann J., Koszinowski U. H. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291:303–305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

