Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling
- PMID: 21813654
- PMCID: PMC3192561
- DOI: 10.1104/pp.111.182931
Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling
Abstract
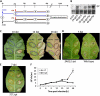
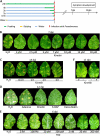
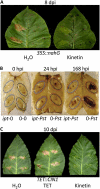
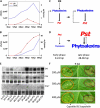
Cytokinins are phytohormones that are involved in various regulatory processes throughout plant development, but they are also produced by pathogens and known to modulate plant immunity. A novel transgenic approach enabling autoregulated cytokinin synthesis in response to pathogen infection showed that cytokinins mediate enhanced resistance against the virulent hemibiotrophic pathogen Pseudomonas syringae pv tabaci. This was confirmed by two additional independent transgenic approaches to increase endogenous cytokinin production and by exogenous supply of adenine- and phenylurea-derived cytokinins. The cytokinin-mediated resistance strongly correlated with an increased level of bactericidal activities and up-regulated synthesis of the two major antimicrobial phytoalexins in tobacco (Nicotiana tabacum), scopoletin and capsidiol. The key role of these phytoalexins in the underlying mechanism was functionally proven by the finding that scopoletin and capsidiol substitute in planta for the cytokinin signal: phytoalexin pretreatment increased resistance against P. syringae. In contrast to a cytokinin defense mechanism in Arabidopsis (Arabidopsis thaliana) based on salicylic acid-dependent transcriptional control, the cytokinin-mediated resistance in tobacco is essentially independent from salicylic acid and differs in pathogen specificity. It is also independent of jasmonate levels, reactive oxygen species, and high sugar resistance. The novel function of cytokinins in the primary defense response of solanaceous plant species is rather mediated through a high phytoalexin-pathogen ratio in the early phase of infection, which efficiently restricts pathogen growth. The implications of this mechanism for the coevolution of host plants and cytokinin-producing pathogens and the practical application in agriculture are discussed.
Figures

Similar articles
-
Abscisic Acid-Cytokinin Antagonism Modulates Resistance Against Pseudomonas syringae in Tobacco.Phytopathology. 2014 Dec;104(12):1283-8. doi: 10.1094/PHYTO-03-14-0076-R. Phytopathology. 2014. PMID: 24941328
-
Structure-function relation of cytokinins determines their differential efficiency in mediating tobacco resistance against Pseudomonas syringae.Physiol Plant. 2025 Jan-Feb;177(1):e70028. doi: 10.1111/ppl.70028. Physiol Plant. 2025. PMID: 39727031 Free PMC article.
-
Phytohormones mediate volatile emissions during the interaction of compatible and incompatible pathogens: the role of ethylene in Pseudomonas syringae infected tobacco.J Chem Ecol. 2005 Mar;31(3):439-59. doi: 10.1007/s10886-005-2018-5. J Chem Ecol. 2005. PMID: 15898494
-
Jasmonic Acid at the Crossroads of Plant Immunity and Pseudomonas syringae Virulence.Int J Mol Sci. 2020 Oct 11;21(20):7482. doi: 10.3390/ijms21207482. Int J Mol Sci. 2020. PMID: 33050569 Free PMC article. Review.
-
Cytokinins and plant immunity: old foes or new friends?Trends Plant Sci. 2011 Jul;16(7):388-94. doi: 10.1016/j.tplants.2011.03.003. Epub 2011 Apr 5. Trends Plant Sci. 2011. PMID: 21470894 Review.
Cited by
-
Integrated systems view on networking by hormones in Arabidopsis immunity reveals multiple crosstalk for cytokinin.Plant Cell. 2012 May;24(5):1793-814. doi: 10.1105/tpc.112.098335. Epub 2012 May 29. Plant Cell. 2012. PMID: 22643121 Free PMC article.
-
Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential.Nutrients. 2022 Apr 2;14(7):1495. doi: 10.3390/nu14071495. Nutrients. 2022. PMID: 35406107 Free PMC article. Review.
-
Probing the unknowns in cytokinin-mediated immune defense in Arabidopsis with systems biology approaches.Bioinform Biol Insights. 2014 Feb 13;8:35-44. doi: 10.4137/BBI.S13462.. eCollection 2014. Bioinform Biol Insights. 2014. PMID: 24558299 Free PMC article.
-
Cytokinin Regulation of Source-Sink Relationships in Plant-Pathogen Interactions.Front Plant Sci. 2021 Aug 24;12:677585. doi: 10.3389/fpls.2021.677585. eCollection 2021. Front Plant Sci. 2021. PMID: 34504504 Free PMC article. Review.
-
Transcriptomic Analysis of Resistant and Susceptible Responses in a New Model Root-Knot Nematode Infection System Using Solanum torvum and Meloidogyne arenaria.Front Plant Sci. 2021 May 25;12:680151. doi: 10.3389/fpls.2021.680151. eCollection 2021. Front Plant Sci. 2021. PMID: 34122492 Free PMC article.
References
-
- Abo-Elyousr K, Mohamed H. (2009) Biological control of Fusarium wilt in tomato by plant growth-promoting yeasts and rhizobacteria hormones produced by Azospirillum brasilense for resistance. Plant Pathol J 25: 199–204
-
- Ahl Goy P, Signer H, Reist R, Aichholz R, Blum W, Schmidt E, Kessmann H. (1993) Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa × Nicotiana debneyi. Planta 191: 200–206
-
- Arkhipova T, Veselov S, Melentiev A, Martynenko E, Kudoyarova G. (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272: 201–209
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

