Suppression of a neocortical potassium channel activity by intracellular amyloid-β and its rescue with Homer1a
- PMID: 21813671
- PMCID: PMC6623357
- DOI: 10.1523/JNEUROSCI.6752-10.2011
Suppression of a neocortical potassium channel activity by intracellular amyloid-β and its rescue with Homer1a
Abstract
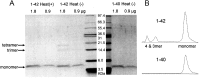
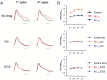
It is proposed that intracellular amyloid-β (Aβ), before extracellular plaque formation, triggers cognitive deficits in Alzheimer disease (AD). Here we report how intracellular Aβ affects neuronal properties. This was done by injecting Aβ protein into rat and mouse neocortical pyramidal cells through whole-cell patch pipettes and by using 3xTg AD model mice, in which intracellular Aβ is accumulated innately. In rats, intracellular application of a mixed Aβ(1-42) preparation containing both oligomers and monomers, but not a monomeric preparation of Aβ(1-40), broadened spike width and augmented Ca(2+) influx via voltage-dependent Ca(2+) channels in neocortical neurons. Both effects were mimicked and occluded by charybdotoxin, a blocker of large-conductance Ca(2+)-activated K(+) (BK) channels, and blocked by isopimaric acid, a BK channel opener. Surprisingly, augmented Ca(2+) influx was caused by elongated spike duration, but not attributable to direct Ca(2+) channel modulation by Aβ(1-42). The Aβ(1-42)-induced spike broadening was blocked by electroconvulsive shock (ECS), which we previously showed to facilitate BK channel opening via expression of the scaffold protein Homer1a. In young 3xTg and wild mice, we confirmed spike broadening by Aβ(1-42), which was again mimicked and occluded by charybdotoxin and blocked by ECS. In Homer1a knock-out mice, ECS failed to block the Aβ(1-42) effect. Single-channel recording on BK channels supported these results. These findings suggest that the suppression of BK channels by intracellular Aβ(1-42) is a possible key mechanism for early dysfunction in the AD brain, which may be counteracted by activity-dependent expression of Homer1a.
Figures







Similar articles
-
Calcium influx via L- and N-type calcium channels activates a transient large-conductance Ca2+-activated K+ current in mouse neocortical pyramidal neurons.J Neurosci. 2003 May 1;23(9):3639-48. doi: 10.1523/JNEUROSCI.23-09-03639.2003. J Neurosci. 2003. PMID: 12736335 Free PMC article.
-
Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer's disease model mice.Neuropharmacology. 2015 Oct;97:210-9. doi: 10.1016/j.neuropharm.2015.05.027. Epub 2015 Jun 5. Neuropharmacology. 2015. PMID: 26051398
-
Essential roles of Homer-1a in homeostatic regulation of pyramidal cell excitability: a possible link to clinical benefits of electroconvulsive shock.Eur J Neurosci. 2005 Jun;21(12):3229-39. doi: 10.1111/j.1460-9568.2005.04165.x. Eur J Neurosci. 2005. PMID: 16026461
-
Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons.J Neurophysiol. 2000 Jan;83(1):70-80. doi: 10.1152/jn.2000.83.1.70. J Neurophysiol. 2000. PMID: 10634854
-
Cognitive recovery by chronic activation of the large-conductance calcium-activated potassium channel in a mouse model of Alzheimer's disease.Neuropharmacology. 2015 May;92:8-15. doi: 10.1016/j.neuropharm.2014.12.033. Epub 2015 Jan 8. Neuropharmacology. 2015. PMID: 25577958
Cited by
-
The Effect of 40-Hz White LED Therapy on Structure-Function of Brain Mitochondrial ATP-Sensitive Ca-Activated Large-Conductance Potassium Channel in Amyloid Beta Toxicity.Neurotox Res. 2022 Oct;40(5):1380-1392. doi: 10.1007/s12640-022-00565-9. Epub 2022 Sep 3. Neurotox Res. 2022. PMID: 36057039
-
Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System.Front Mol Neurosci. 2018 Jul 30;11:258. doi: 10.3389/fnmol.2018.00258. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30104956 Free PMC article. Review.
-
The Complex Formed by Group I Metabotropic Glutamate Receptor (mGluR) and Homer1a Plays a Central Role in Metaplasticity and Homeostatic Synaptic Scaling.J Neurosci. 2021 Jun 30;41(26):5567-5578. doi: 10.1523/JNEUROSCI.0026-21.2021. J Neurosci. 2021. PMID: 34193623 Free PMC article. Review.
-
The Role of Astrocytes in the Central Nervous System Focused on BK Channel and Heme Oxygenase Metabolites: A Review.Antioxidants (Basel). 2019 May 5;8(5):121. doi: 10.3390/antiox8050121. Antioxidants (Basel). 2019. PMID: 31060341 Free PMC article. Review.
-
Amyloid β and Amyloid Precursor Protein Synergistically Suppress Large-Conductance Calcium-Activated Potassium Channel in Cortical Neurons.Front Aging Neurosci. 2021 Jun 3;13:660319. doi: 10.3389/fnagi.2021.660319. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34149396 Free PMC article.
References
-
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. - PubMed
-
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. - PubMed
-
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
