Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development
- PMID: 21813673
- PMCID: PMC3158567
- DOI: 10.1523/JNEUROSCI.1709-11.2011
Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development
Abstract
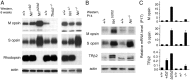
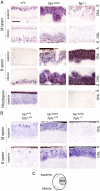
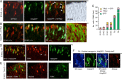
The typical mammalian visual system is based upon three photoreceptor types: rods for dim light vision and two types of cones (M and S) for color vision in daylight. However, the process that generates photoreceptor diversity and the cell type in which diversity arises remain unclear. Mice deleted for thyroid hormone receptor β2 (TRβ2) and neural retina leucine zipper factor (NRL) lack M cones and rods, respectively, but gain S cones. We therefore tested the hypothesis that NRL and TRβ2 direct a common precursor to a rod, M cone, or S cone outcome using Nrl(b2/b2) "knock-in" mice that express TRβ2 instead of NRL from the endogenous Nrl gene. Nrl(b2/b2) mice lacked rods and produced excess M cones in contrast to the excess S cones in Nrl(-/-) mice. Notably, the presence of both factors yielded rods in Nrl(+/b2) mice. The results demonstrate innate plasticity in postmitotic rod precursors that allows these cells to form three functional photoreceptor types in response to NRL or TRβ2. We also detected precursor cells in normal embryonic retina that transiently coexpressed Nrl and TRβ2, suggesting that some precursors may originate in a plastic state. The plasticity of the precursors revealed in Nrl(b2/b2) mice suggests that a two-step transcriptional switch can direct three photoreceptor fates: first, rod versus cone identity dictated by NRL, and second, if NRL fails to act, M versus S cone identity dictated by TRβ2.
Figures



References
-
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. - PubMed
-
- Akimoto M, Cheng H, Zhu D, Brzezinski JA, Khanna R, Filippova E, Oh EC, Jing Y, Linares JL, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci U S A. 2006;103:3890–3895. - PMC - PubMed
-
- Alfano G, Conte I, Caramico T, Avellino R, Arnò B, Pizzo MT, Tanimoto N, Beck SC, Huber G, Dollé P, Seeliger MW, Banfi S. Vax2 regulates retinoic acid distribution and cone opsin expression in the vertebrate eye. Development. 2011;138:261–271. - PubMed
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
