Visual-induced excitation leads to firing pauses in striatal cholinergic interneurons
- PMID: 21813675
- PMCID: PMC6623370
- DOI: 10.1523/JNEUROSCI.0661-11.2011
Visual-induced excitation leads to firing pauses in striatal cholinergic interneurons
Abstract
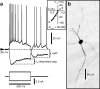
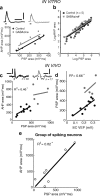
Tonically active neurons in the primate striatum, believed to be cholinergic interneurons (CINs), respond to sensory stimuli with a pronounced pause in firing. Although inhibitory and neuromodulatory mechanisms have been implicated, it is not known how sensory stimuli induce firing pauses in CINs in vivo. Here, we used intracellular recordings in anesthetized rats to investigate the effectiveness of a visual stimulus at modulating spike activity in CINs. Initially, no neuron was visually responsive. However, following pharmacological activation of tecto-thalamic pathways, the firing pattern of most CINs was significantly modulated by a light flashed into the contralateral eye. Typically, this induced an excitation followed by a pause in spike firing, via an underlying depolarization-hyperpolarization membrane sequence. Stimulation of thalamic afferents in vitro evoked similar responses that were independent of synaptic inhibition. Thus, visual stimulation likely induces an initial depolarization via a subcortical tecto-thalamo-striatal pathway, pausing CIN firing through an intrinsic afterhyperpolarization.
Figures








References
-
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
