Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease
- PMID: 21813677
- PMCID: PMC6623351
- DOI: 10.1523/JNEUROSCI.6209-10.2011
Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease
Abstract
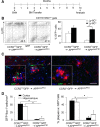
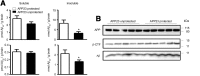
Mononuclear phagocytes are important modulators of Alzheimer's disease (AD), but the specific functions of resident microglia, bone marrow-derived mononuclear cells, and perivascular macrophages have not been resolved. To elucidate the spatiotemporal roles of mononuclear phagocytes during disease, we targeted myeloid cell subsets from different compartments and examined disease pathogenesis in three different mouse models of AD (APP(swe/PS1), APP(swe), and APP23 mice). We identified chemokine receptor 2 (CCR2)-expressing myeloid cells as the population that was preferentially recruited to β-amyloid (Aβ) deposits. Unexpectedly, AD brains with dysfunctional microglia and devoid of parenchymal bone marrow-derived phagocytes did not show overt changes in plaque pathology and Aβ load. In contrast, restriction of CCR2 deficiency to perivascular myeloid cells drastically impaired β-amyloid clearance and amplified vascular Aβ deposition, while parenchymal plaque deposition remained unaffected. Together, our data advocate selective functions of CCR2-expressing myeloid subsets, which could be targeted specifically to modify disease burden in AD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Algotsson A, Winblad B. The integrity of the blood–brain barrier in Alzheimer's disease. Acta Neurol Scand. 2007;115:403–408. - PubMed
-
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
