Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey
- PMID: 21813682
- PMCID: PMC3183456
- DOI: 10.1523/JNEUROSCI.0257-11.2011
Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey
Abstract
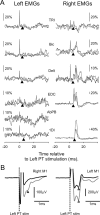
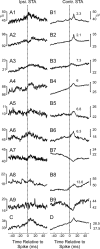
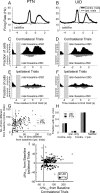
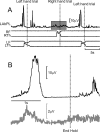
Strong experimental evidence implicates the corticospinal tract in voluntary control of the contralateral forelimb. Its potential role in controlling the ipsilateral forelimb is less well understood, although anatomical projections to ipsilateral spinal circuits are identified. We investigated inputs to motoneurons innervating hand and forearm muscles from the ipsilateral corticospinal tract using multiple methods. Intracellular recordings from 62 motoneurons in three anesthetized monkeys revealed no monosynaptic and only one weak oligosynaptic EPSP after stimulation of the ipsilateral corticospinal tract. Single stimulus intracortical microstimulation of the primary motor cortex (M1) in awake animals failed to produce any responses in ipsilateral muscles. Strong stimulation (>500 μA, single stimulus) of the majority of corticospinal axons at the medullary pyramids revealed only weak suppressions in ipsilateral muscles at longer latencies than the robust facilitations seen contralaterally. Spike-triggered averaging of ipsilateral muscle activity from M1 neural discharge (184 cells) did not reveal any postspike effects consistent with monosynaptic corticomotoneuronal connections. We also examined the activity of 191 M1 neurons during ipsilateral or contralateral "reach to precision grip" movements. Many cells (67%) modulated their activity during ipsilateral limb movement trials (compared with 90% with contralateral trials), but the timing of this activity was best correlated with weak muscle activity in the contralateral nonmoving arm. We conclude that, in normal adults, any inputs to forelimb motoneurons from the ipsilateral corticospinal tract are weak and indirect and that modulation of M1 cell firing seems to be related primarily to control of the contralateral limb.
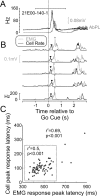
Figures



References
-
- Aizawa H, Mushiake H, Inase M, Tanji J. An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Exp Brain Res. 1990;82:219–221. - PubMed
-
- Armatas CA, Summers JJ, Bradshaw JL. Mirror movements in normal adult subjects. J Clin Exp Neuropsychol. 1994;16:405–413. - PubMed
-
- Baker SN, Lemon RN. A computer simulation study of the production of post-spike facilitation in spike triggered averages of rectified EMG. J Neurophysiol. 1998;80:1391–1406. - PubMed
-
- Baker SN, Lemon RN. Precise spatiotemporal repeating patterns in monkey primary and supplementary motor areas occur at chance levels. J Neurophysiol. 2000;84:1770–1780. - PubMed
-
- Baker SN, Olivier E, Lemon RN. An investigation of the intrinsic circuitry of the motor cortex of the monkey using intra-cortical microstimulation. Exp Brain Res. 1998;123:397–411. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
