Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning
- PMID: 21813688
- PMCID: PMC6623364
- DOI: 10.1523/JNEUROSCI.1810-11.2011
Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning
Abstract
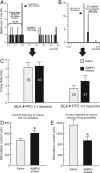
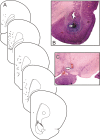
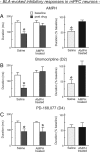
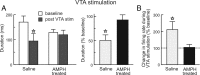
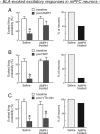
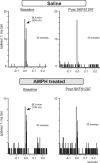
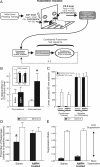
Repeated exposure to psychostimulants such as amphetamine (AMPH) disrupts cognitive and behavioral processes mediated by the medial prefrontal cortical (mPFC) and basolateral amygdala (BLA). The present study investigated the effects of repeated AMPH exposure on the neuromodulatory actions of dopamine (DA) on BLA-mPFC circuitry and cognitive/emotional processing mediated by these circuits. Rats received five AMPH (2 mg/kg) or saline injections (controls) over 10 d, followed by 2-4 week drug washout. In vivo neurophysiological extracellular recordings in urethane-anesthetized rats were used to obtain data from mPFC neurons that were either inhibited or excited by BLA stimulation. In controls, acute AMPH attenuated BLA-evoked inhibitory or excitatory responses; these effects were mimicked by selective D(2) or D(1) agonists, respectively. However, in AMPH-treated rats, the ability of these dopaminergic manipulations to modulate BLA-driven decreases/increases in mPFC activity was abolished. Repeated AMPH also blunted the excitatory effects of ventral tegmental area stimulation on mPFC neural firing. Behavioral studies assessed the effect of repeated AMPH on decision making with conditioned punishment, a process mediated by BLA-mPFC circuitry and mesocortical DA. These treatments impaired the ability of rats to use conditioned aversive stimuli (footshock-associated cue) to guide the direction of instrumental responding. Collectively, these data suggest that repeated AMPH exposure can lead to persistent disruption of dopaminergic modulation of BLA-mPFC circuitry, which may underlie impairments in cognitive/emotional processing observed in stimulant abusers. Furthermore, they suggest that impairments in decision making guided by aversive stimuli observed in stimulant abusers may be the result of repeated drug exposure.
Figures

References
-
- Au-Young SM, Shen H, Yang CR. Medial prefrontal cortical output neurons to the ventral tegmental area (VTA) and their responses to burst-patterned stimulation of the VTA: neuroanatomical and in vivo electrophysiological analyses. Synapse. 1999;34:245–255. - PubMed
-
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. - PubMed
-
- Bolles RC, Uhl CN, Wolfe M, Chase PB. Stimulus learning versus response learning in a discriminated punishment situation. Learn Motiv. 1975;6:439–444.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
