The detection of visual contrast in the behaving mouse
- PMID: 21813694
- PMCID: PMC6623377
- DOI: 10.1523/JNEUROSCI.6689-10.2011
The detection of visual contrast in the behaving mouse
Abstract
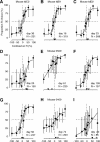
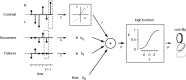
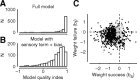
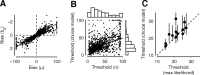
The mouse is becoming a key species for research on the neural circuits of the early visual system. To relate such circuits to perception, one must measure visually guided behavior and ask how it depends on fundamental stimulus attributes such as visual contrast. Using operant conditioning, we trained mice to detect visual contrast in a two-alternative forced-choice task. After 3-4 weeks of training, mice performed hundreds of trials in each session. Numerous sessions yielded high-quality psychometric curves from which we inferred measures of contrast sensitivity. In multiple sessions, however, choices were influenced not only by contrast, but also by estimates of reward value and by irrelevant factors such as recent failures and rewards. This behavior was captured by a generalized linear model involving not only the visual responses to the current stimulus but also a bias term and history terms depending on the outcome of the previous trial. We compared the behavioral performance of the mice to predictions of a simple decoder applied to neural responses measured in primary visual cortex of awake mice during passive viewing. The decoder performed better than the animal, suggesting that mice might not use optimally the information contained in the activity of visual cortex.
Figures






References
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. - PubMed
-
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–366. - PubMed
-
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
