Trs65p, a subunit of the Ypt1p GEF TRAPPII, interacts with the Arf1p exchange factor Gea2p to facilitate COPI-mediated vesicle traffic
- PMID: 21813735
- PMCID: PMC3183018
- DOI: 10.1091/mbc.E11-03-0197
Trs65p, a subunit of the Ypt1p GEF TRAPPII, interacts with the Arf1p exchange factor Gea2p to facilitate COPI-mediated vesicle traffic
Abstract
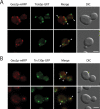
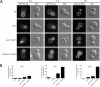
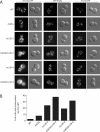
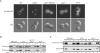
The TRAPP complexes are multimeric guanine exchange factors (GEFs) for the Rab GTPase Ypt1p. The three complexes (TRAPPI, TRAPPII, and TRAPPIII) share a core of common subunits required for GEF activity, as well as unique subunits (Trs130p, Trs120p, Trs85p, and Trs65p) that redirect the GEF from the endoplasmic reticulum-Golgi pathway to different cellular locations where TRAPP mediates distinct membrane trafficking events. Roles for three of the four unique TRAPP subunits have been described before; however, the role of the TRAPPII-specific subunit Trs65p has remained elusive. Here we demonstrate that Trs65p directly binds to the C-terminus of the Arf1p exchange factor Gea2p and provide in vivo evidence that this interaction is physiologically relevant. Gea2p and TRAPPII also bind to the yeast orthologue of the γ subunit of the COPI coat complex (Sec21p), a known Arf1p effector. These and previous findings reveal that TRAPPII is part of an Arf1p GEF-effector loop that appears to play a role in recruiting or stabilizing TRAPPII to membranes. In support of this proposal, we show that TRAPPII is more soluble in an arf1Δ mutant.
Figures



References
-
- Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–763. - PubMed
-
- Beck R, Adolf F, Weimer C, Bruegger B, Wieland FT. ArfGAP1 activity and COPI vesicle biogenesis. Traffic. 2009;10:307–315. - PubMed
-
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. - PubMed
-
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007a;12:671–682. - PubMed
-
- Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007b;445:941–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

