MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP
- PMID: 21813776
- PMCID: PMC3159690
- DOI: 10.4049/jimmunol.1100088
MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP
Abstract
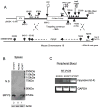
Cyclic-di-GMP and cyclic-di-AMP are second messengers produced by bacteria and influence bacterial cell survival, differentiation, colonization, biofilm formation, virulence, and bacteria-host interactions. In this study, we show that in both RAW264.7 macrophage cells and primary bone marrow-derived macrophages, the production of IFN-β and IL-6, but not TNF, in response to cyclic-di-AMP and cyclic-di-GMP requires MPYS (also known as STING, MITA, and TMEM173). Furthermore, expression of MPYS was required for IFN response factor 3 but not NF-κB activation in response to these bacterial metabolites. We also confirm that MPYS is required for type I IFN production by cultured macrophages infected with the intracellular pathogens Listeria monocytogenes and Francisella tularensis. However, during systemic infection with either pathogen, MPYS deficiency did not impact bacterial burdens in infected spleens. Serum IFN-β and IL-6 concentrations in the infected control and MPYS(-/-) mice were also similar at 24 h postinfection, suggesting that these pathogens stimulate MPYS-independent cytokine production during in vivo infection. Our findings indicate that bifurcating MPYS-dependent and -independent pathways mediate sensing of cytosolic bacterial infections.
Figures




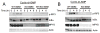
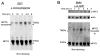

References
-
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. - PubMed
-
- Dinarello CA. Anti-cytokine therapeutics and infections. Vaccine. 2003;21(Suppl 2):S24–34. - PubMed
-
- Raychaudhuri SP, Nguyen CT, Raychaudhuri SK, Gershwin ME. Incidence and nature of infectious disease in patients treated with anti-TNF agents. Autoimmun Rev. 2009;9:67–81. - PubMed
-
- Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. - PubMed
-
- Freudenberg MA, Merlin T, Kalis C, Chvatchko Y, Stubig H, Galanos C. Cutting edge: a murine, IL-12-independent pathway of IFN-gamma induction by gram-negative bacteria based on STAT4 activation by Type I IFN and IL-18 signaling. J Immunol. 2002;169:1665–1668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

