Phase II screening trial of lithium carbonate in amyotrophic lateral sclerosis: examining a more efficient trial design
- PMID: 21813790
- PMCID: PMC3171956
- DOI: 10.1212/WNL.0b013e31822dc7a5
Phase II screening trial of lithium carbonate in amyotrophic lateral sclerosis: examining a more efficient trial design
Abstract
Objective: To use a historical placebo control design to determine whether lithium carbonate slows progression of amyotrophic lateral sclerosis (ALS).
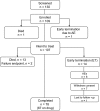
Methods: A phase II trial was conducted at 10 sites in the Western ALS Study Group using similar dosages (300-450 mg/day), target blood levels (0.3-0.8 mEq/L), outcome measures, and trial duration (13 months) as the positive trial. However, taking riluzole was not a requirement for study entry. Placebo outcomes in patients matched for baseline features from a large database of recent clinical trials, showing stable rates of decline over the past 9 years, were used as historical controls.
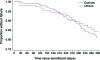
Results: The mean rate of decline of the ALS Functional Rating Scale-Revised was greater in 107 patients taking lithium carbonate (-1.20/month, 95% confidence interval [CI] -1.41 to -0.98) than that in 249 control patients (-1.01/month, 95% CI -1.11 to -0.92, p = 0.04). There were no differences in secondary outcome measures (forced vital capacity, time to failure, and quality of life), but there were more adverse events in the treated group.
Conclusions: The lack of therapeutic benefit and safety concerns, taken together with similar results from 2 other recent trials, weighs against the use of lithium carbonate in patients with ALS. The absence of drift over time and the availability of a large database of patients for selecting a matched historical control group suggest that use of historical controls may result in more efficient phase II trials for screening putative ALS therapeutic agents.
Classification of evidence: This study provided Class IV evidence that lithium carbonate does not slow the rate of decline of function in patients with ALS over 13 months.
Figures

Comment in
-
Historical controls in ALS trials: a high seas rescue?Neurology. 2011 Sep 6;77(10):936-7. doi: 10.1212/WNL.0b013e31822cfcb6. Epub 2011 Aug 3. Neurology. 2011. PMID: 21813782 No abstract available.
-
Lithium treatment in amyotrophic lateral sclerosis: do we have enough trials?Expert Rev Neurother. 2011 Dec;11(12):1693-8. doi: 10.1586/ern.11.164. Expert Rev Neurother. 2011. PMID: 22091594
References
-
- Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1218–1226 - PMC - PubMed
-
- Fornai F, Longone P, Ferrucci M, et al. Autophagy and amyotrophic lateral sclerosis: the multiple roles of lithium. Autophagy 2008;4:527–530 - PubMed
-
- Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy 2006;2:132–134 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
