Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes
- PMID: 21813803
- PMCID: PMC3178299
- DOI: 10.2337/db11-0538
Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes
Abstract
Objective: Neonatal diabetes mellitus (NDM) can be caused by gain-of-function ATP-sensitive K(+) (K(ATP)) channel mutations. This realization has led to sulfonylurea therapy replacing insulin injections in many patients. In a murine model of K(ATP)-dependent NDM, hyperglycemia and consequent loss of β-cells are both avoided by chronic sulfonylurea treatment. Interestingly, K(ATP) mutations may underlie remitting-relapsing, transient, or permanent forms of the disease in different patients, but the reason for the different outcomes is unknown.
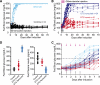
Research design and methods: To gain further insight into disease progression and outcome, we examined the effects of very early intervention by injecting NDM mice with high-dose glibenclamide for only 6 days, at the beginning of disease onset, then after the subsequent progression with measurements of blood glucose, islet function, and insulin sensitivity.
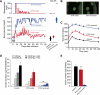
Results: Although ∼70% of mice developed severe diabetes after treatment cessation, ∼30% were essentially cured, maintaining near-normal blood glucose until killed. Another group of NDM mice was initiated on oral glibenclamide (in the drinking water), and the dose was titrated daily, to maintain blood glucose <200 mg/dL. In this case, ∼30% were also essentially cured; they were weaned from the drug after ∼4 weeks and again subsequently maintained near-normal blood glucose. These cured mice maintain normal insulin content and were more sensitive to insulin than control mice, a compensatory mechanism that together with basal insulin secretion may be sufficient to maintain near-normal glucose levels.
Conclusions: At least in a subset of animals, early sulfonylurea treatment leads to permanent remission of NDM. These cured animals exhibit insulin-hypersensitivity. Although untreated NDM mice rapidly lose insulin content and progress to permanently extremely elevated blood glucose levels, early tight control of blood glucose may permit this insulin-hypersensitivity, in combination with maintained basal insulin secretion, to provide long-term remission.
Figures

References
-
- Clement JP, 4th, Kunjilwar K, Gonzalez G, et al. Association and stoichiometry of K(ATP) channel subunits. Neuron 1997;18:827–838 - PubMed
-
- Inagaki N, Gonoi T, Clement JP, 4th, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 1995;270:1166–1170 - PubMed
-
- Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350:1838–1849 - PubMed
-
- Hamilton-Shield JP. Overview of neonatal diabetes. Endocr Dev 2007;12:12–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

