DNA mismatch repair proficiency executing 5-fluorouracil cytotoxicity in colorectal cancer cells
- PMID: 21814034
- PMCID: PMC3367669
- DOI: 10.4161/cbt.12.8.17169
DNA mismatch repair proficiency executing 5-fluorouracil cytotoxicity in colorectal cancer cells
Abstract
Background: 5-fluorouracil (5FU)-based chemotherapy is the standard treatment for advanced stage colorectal cancer (CRC) patients. Several groups including ours have reported that stage II-III colorectal cancer patients whose tumors retain DNA Mismatch repair (MMR) function derive a benefit from 5FU, but patients with tumors that lost MMR function do not. Although MMR recognition of 5FU incorporated in DNA has been demonstrated biochemically, it has not been demonstrated within cells to execute 5FU cytotoxicity.
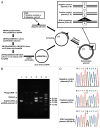
Aim: To establish an efficient construction model for 5FU within DNA and demonstrate that 5FU incorporated into DNA can trigger cellular cytotoxicity executed by the DNA MMR system.
Methods: We constructed a 5FdU-containing heteroduplex plasmid (5FdU plasmid) and 5FdU-containing linear dsDNA (5FdU linear DNA), and transfected these into MMR-proficient, hMLH1-/- and hMSH6-/- cells. We observed cell growth characteristics of both transfectants for 5FU-induced cytotoxicity.
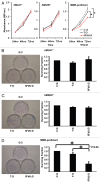
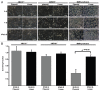
Results: MMR- proficient cells transfected with the 5FdU plasmid but not the 5FdU linear DNA showed reduced cell proliferation by MTS and clonogenic assays, and demonstrated cell morphological change consistent with apoptosis. In MMR-deficient cells, neither the 5FdU plasmid nor 5FdU linear DNA induced cell growth or morphological changes different from controls.
Conclusion: 5FdU as heteroduplex DNA in plasmid but not linear form triggered cytotoxicity in a MMR-dependent manner. Thus 5FU incorporated into DNA, separated from its effects on RNA, can be recognized by DNA MMR to trigger cell death.
Figures

References
-
- Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989;7:1447–1456. - PubMed
-
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–326. - PubMed
-
- Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol. 1998;16:301–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
