On the growth and form of the gut
- PMID: 21814276
- PMCID: PMC3335276
- DOI: 10.1038/nature10277
On the growth and form of the gut
Abstract
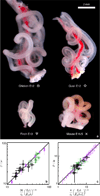
The developing vertebrate gut tube forms a reproducible looped pattern as it grows into the body cavity. Here we use developmental experiments to eliminate alternative models and show that gut looping morphogenesis is driven by the homogeneous and isotropic forces that arise from the relative growth between the gut tube and the anchoring dorsal mesenteric sheet, tissues that grow at different rates. A simple physical mimic, using a differentially strained composite of a pliable rubber tube and a soft latex sheet is consistent with this mechanism and produces similar patterns. We devise a mathematical theory and a computational model for the number, size and shape of intestinal loops based solely on the measurable geometry, elasticity and relative growth of the tissues. The predictions of our theory are quantitatively consistent with observations of intestinal loops at different stages of development in the chick embryo. Our model also accounts for the qualitative and quantitative variation in the distinct gut looping patterns seen in a variety of species including quail, finch and mouse, illuminating how the simple macroscopic mechanics of differential growth drives the morphology of the developing gut.
Figures




Comment in
-
Development: Predictable looping of the developing vertebrate gut explained.Nat Rev Gastroenterol Hepatol. 2011 Oct 5;8(10):533. doi: 10.1038/nrgastro.2011.143. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21970956 No abstract available.
References
-
- His W. Anatomie Menschlicher Embryonen. Leipzig, Germany: Verlag von F.C.W. Vogel; 1880.
-
- Thompson DW. On Growth and Form. Cambridge: Cambridge University Press; 1917.
-
- Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell. 1997;90:979–990. - PubMed
-
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

