Nickel-Catalyzed Coupling Reactions of Alkenes
- PMID: 21814295
- PMCID: PMC3148197
- DOI: 10.1351/pac200880050929
Nickel-Catalyzed Coupling Reactions of Alkenes
Abstract
Several reactions of simple, unactivated alkenes with electrophiles under nickel(0) catalysis are discussed. The coupling of olefins with aldehydes and silyl triflates provides allylic or homoallylic alcohol derivatives, depending on the supporting ligands and, to a lesser extent, the substrates employed. Reaction of alkenes with isocyanates yields N-alkyl acrylamides. In these methods, alkenes act as the functional equivalents of alkenyl- and allylmetal reagents.
Figures
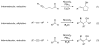
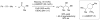








References
-
- Lappin GR, Sauer JD. Alpha Olefins Applications Handbook. Marcel Dekker; New York: 1989.
-
- Oblinger E, Montgomery J. J Am Chem Soc. 1997;119:9065.
-
- Huang WS, Chan J, Jamison TF. Org Lett. 2000;2:4221. - PubMed
-
- Miller KM, Huang WS, Jamison TF. J Am Chem Soc. 2003;125:3442. - PubMed
- Van Dyke AR, Miller KM, Jamison TF. Org Synth. 2007;84:111.
Grants and funding
LinkOut - more resources
Full Text Sources
