A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection
- PMID: 21814510
- PMCID: PMC3141034
- DOI: 10.1371/journal.ppat.1002098
A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection
Abstract
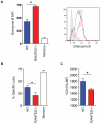
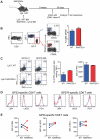
RANTES (CCL5) is a chemokine expressed by many hematopoietic and non-hematopoietic cell types that plays an important role in homing and migration of effector and memory T cells during acute infections. The RANTES receptor, CCR5, is a major target of anti-HIV drugs based on blocking viral entry. However, defects in RANTES or RANTES receptors including CCR5 can compromise immunity to acute infections in animal models and lead to more severe disease in humans infected with west Nile virus (WNV). In contrast, the role of the RANTES pathway in regulating T cell responses and immunity during chronic infection remains unclear. In this study, we demonstrate a crucial role for RANTES in the control of systemic chronic LCMV infection. In RANTES⁻/⁻ mice, virus-specific CD8 T cells had poor cytokine production. These RANTES⁻/⁻ CD8 T cells also expressed higher amounts of inhibitory receptors consistent with more severe exhaustion. Moreover, the cytotoxic ability of CD8 T cells from RANTES⁻/⁻ mice was reduced. Consequently, viral load was higher in the absence of RANTES. The dysfunction of T cells in the absence of RANTES was as severe as CD8 T cell responses generated in the absence of CD4 T cell help. Our results demonstrate an important role for RANTES in sustaining CD8 T cell responses during a systemic chronic viral infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. - PubMed
-
- Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol. 2005;125:615–628. - PubMed
-
- Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156:322–327. - PubMed
-
- Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

