Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts
- PMID: 21814580
- PMCID: PMC3141042
- DOI: 10.1371/journal.pone.0022453
Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts
Abstract
Background: Despite over forty years of investigation on low-level light therapy (LLLT), the fundamental mechanisms underlying photobiomodulation at a cellular level remain unclear.
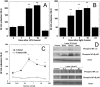
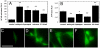
Methodology/principal findings: In this study, we isolated murine embryonic fibroblasts (MEF) from transgenic NF-kB luciferase reporter mice and studied their response to 810 nm laser radiation. Significant activation of NF-kB was observed at fluences higher than 0.003 J/cm(2) and was confirmed by Western blot analysis. NF-kB was activated earlier (1 hour) by LLLT compared to conventional lipopolysaccharide treatment. We also observed that LLLT induced intracellular reactive oxygen species (ROS) production similar to mitochondrial inhibitors, such as antimycin A, rotenone and paraquat. Furthermore, we observed similar NF-kB activation with these mitochondrial inhibitors. These results, together with inhibition of laser induced NF-kB activation by antioxidants, suggests that ROS play an important role in the laser induced NF-kB signaling pathways. However, LLLT, unlike mitochondrial inhibitors, induced increased cellular ATP levels, which indicates that LLLT also upregulates mitochondrial respiration.
Conclusion: We conclude that LLLT not only enhances mitochondrial respiration, but also activates the redox-sensitive NFkB signaling via generation of ROS. Expression of anti-apoptosis and pro-survival genes responsive to NFkB could explain many clinical effects of LLLT.
Conflict of interest statement
Figures


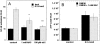
Similar articles
-
Molecular signaling and mechanisms of low-level laser-induced gene expression in cells involved in orthodontic tooth movement.J Formos Med Assoc. 2024 Apr;123(4):442-451. doi: 10.1016/j.jfma.2023.09.011. Epub 2023 Oct 5. J Formos Med Assoc. 2024. PMID: 37805307
-
Effect of photobiomodulation on CCC-ESF reactive oxygen species steady-state in high glucose mediums.Lasers Med Sci. 2021 Apr;36(3):555-562. doi: 10.1007/s10103-020-03057-4. Epub 2020 Jul 9. Lasers Med Sci. 2021. PMID: 32643032
-
Modulation of lipopolysaccharide-induced NF-κB signaling pathway by 635 nm irradiation via heat shock protein 27 in human gingival fibroblast cells.Photochem Photobiol. 2013 Jan-Feb;89(1):199-207. doi: 10.1111/j.1751-1097.2012.01225.x. Epub 2012 Sep 18. Photochem Photobiol. 2013. PMID: 22892019
-
Photobiomodulation CME part I: Overview and mechanism of action.J Am Acad Dermatol. 2024 Nov;91(5):793-802. doi: 10.1016/j.jaad.2023.10.073. Epub 2024 Feb 1. J Am Acad Dermatol. 2024. PMID: 38309304 Review.
-
Role of low-level laser therapy in neurorehabilitation.PM R. 2010 Dec;2(12 Suppl 2):S292-305. doi: 10.1016/j.pmrj.2010.10.013. PM R. 2010. PMID: 21172691 Free PMC article. Review.
Cited by
-
Shining light on nanotechnology to help repair and regeneration.Biotechnol Adv. 2013 Sep-Oct;31(5):607-31. doi: 10.1016/j.biotechadv.2012.08.003. Epub 2012 Aug 21. Biotechnol Adv. 2013. PMID: 22951919 Free PMC article. Review.
-
Noninvasive Systemic Modalities for Prevention of Head and Neck Radiation-Associated Soft Tissue Injury: A Narrative Review.J Reconstr Microsurg. 2022 Oct;38(8):621-629. doi: 10.1055/s-0042-1742731. Epub 2022 Feb 25. J Reconstr Microsurg. 2022. PMID: 35213927 Free PMC article. Review.
-
Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70.Int J Mol Sci. 2022 May 7;23(9):5221. doi: 10.3390/ijms23095221. Int J Mol Sci. 2022. PMID: 35563611 Free PMC article. Review.
-
Efficacy of 1064 nm Photobiomodulation Dosimetry Delivered with a Collimated Flat-Top Handpiece in the Management of Peripheral Facial Paralysis in Patients Unresponsive to Standard Treatment Care: A Case Series.J Clin Med. 2023 Sep 29;12(19):6294. doi: 10.3390/jcm12196294. J Clin Med. 2023. PMID: 37834941 Free PMC article.
-
Mitochondria as a target for neuroprotection: role of methylene blue and photobiomodulation.Transl Neurodegener. 2020 Jun 1;9(1):19. doi: 10.1186/s40035-020-00197-z. Transl Neurodegener. 2020. PMID: 32475349 Free PMC article. Review.
References
-
- Tunér J, Hode L. Laser therapy, clinical practice and scientific background. In: Grängesberg, editor. Sweden: Prima Books; 2002.
-
- Karu TI. London, UK: Gordon and Breach Scientific Publications; 1998. The science of low power laser therapy.
-
- Karu T. Photobiology of low-power laser effects. Health Phys. 1989;56:691–704. - PubMed
-
- Szundi I, Liao GL, Einarsdottir O. Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen. Biochemistry. 2001;40:2332–2339. - PubMed
-
- Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol. 2000;76:863–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

