The importance of tissue segmentation for dose calculations for kilovoltage radiation therapy
- PMID: 21815377
- PMCID: PMC3125081
- DOI: 10.1118/1.3589138
The importance of tissue segmentation for dose calculations for kilovoltage radiation therapy
Abstract
Purpose: The aim of this work was to evaluate the effect of tissue segmentation on the accuracy of Monte Carlo (MC) dose calculations for kilovoltage radiation therapy, which are commonly used in preclinical radiotherapy studies and are also being revisited as a clinical treatment modality. The feasibility of tissue segmentation routinely done on the basis of differences in tissue mass densities was studied and a new segmentation scheme based on differences in effective atomic numbers was developed.
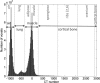
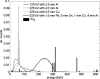
Methods: MC dose calculations in a cylindrical mouse phantom with small cylindrical inhomogeneities consisting of 34 ICRU-44 tissues were performed using the EGSnrc/BEAMnrc and DOSXYZnrc codes. The dose to tissue was calculated for five different kilovoltage beams currently used in small animal radiotherapy: a microCT 120 kV beam, two 225 kV beams filtered with either 4 mm of Al or 0.5 mm of Cu, a heavily filtered 320 kV beam, and a 192Ir beam. The mean doses to the 34 ICRU-44 tissues as a function of tissue mass density and effective atomic number and beam energy were studied. A treatment plan for an orthotopic lung tumor model was created, and the dose distribution was calculated for three tissue segmentation schemes using 4, 8, and 39 tissue bins to assess the significance of the simulation results for kilovoltage radiotherapy.
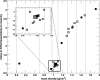
Results: In our model, incorrect assignment of adipose tissue to muscle caused dose calculation differences of 27%, 13%, and 7% for the 120 kV beam and the 225 kV beams filtered with 4 mm Al and 0.5 mm Cu, respectively. For the heavily filtered 320 kV beam and a 192Ir source, potential dose calculation differences due to tissue mis-assignment were below 4%. There was no clear relationship between the dose to tissue and its mass density for x-ray beams generated by tube potentials equal or less than 225 kV. A second order polynomial fit approximated well the absorbed dose to tissue as a function of effective atomic number for these beams. In the mouse study, the 120 kV beam dose to bone was overestimated by 100% and underestimated by 10% for the 4 and 8-tissue segmentation schemes compared to the 39-tissue segmentation scheme, respectively. Dose to adipose tissue was overestimated by 30% and underestimated by 10%, respectively. In general, organ at risk (OAR) doses were overestimated in the 4-tissue and the 8-tissue segmentation schemes compared to the 39-tissue segmentation.
Conclusions: Tissue segmentation was shown to be a key parameter for dose calculations with kilovoltage beams used in small animal radiotherapy when an x-ray tube with a potential < or = 225 kV is used as a source. A new tissue segmentation scheme with 39 tissues based on effective number differences derived from mass density differences has been implemented.
Figures



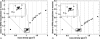




References
-
- Hillman G. G., Maughan R. L., Grignon D. J., Yudelev M., Rubio J.,Tekyi-Mensah S., Layer A., Che M. X., and Forman J. D., “Neutron or photon irradiation for prostate tumors: Enhancement of cytokine therapy in a metastatic tumor model,” Clin. Cancer Res. 7, 136–144 (2001). - PubMed
-
- Wong J., Armour E., Kazanzides P., Iordachita U., Tryggestad E., Deng H., Matinfar M., Kennedy C., Liu Z. J., Chan T., Gray O., Verhaegen F., McNutt T., Ford E., and DeWeese T. L., “High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities,” Int. J.Radiat. Oncol. Biol. Phys. 71, 1591–1599 (2008).10.1016/j.ijrobp.2008.04.025 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

