Assessment of severe malaria in a multicenter, phase III, RTS, S/AS01 malaria candidate vaccine trial: case definition, standardization of data collection and patient care
- PMID: 21816031
- PMCID: PMC3221632
- DOI: 10.1186/1475-2875-10-221
Assessment of severe malaria in a multicenter, phase III, RTS, S/AS01 malaria candidate vaccine trial: case definition, standardization of data collection and patient care
Abstract
Background: An effective malaria vaccine, deployed in conjunction with other malaria interventions, is likely to substantially reduce the malaria burden. Efficacy against severe malaria will be a key driver for decisions on implementation. An initial study of an RTS, S vaccine candidate showed promising efficacy against severe malaria in children in Mozambique. Further evidence of its protective efficacy will be gained in a pivotal, multi-centre, phase III study. This paper describes the case definitions of severe malaria used in this study and the programme for standardized assessment of severe malaria according to the case definition.
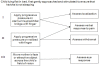
Methods: Case definitions of severe malaria were developed from a literature review and a consensus meeting of expert consultants and the RTS, S Clinical Trial Partnership Committee, in collaboration with the World Health Organization and the Malaria Clinical Trials Alliance. The same groups, with input from an Independent Data Monitoring Committee, developed and implemented a programme for standardized data collection.The case definitions developed reflect the typical presentations of severe malaria in African hospitals. Markers of disease severity were chosen on the basis of their association with poor outcome, occurrence in a significant proportion of cases and on an ability to standardize their measurement across research centres. For the primary case definition, one or more clinical and/or laboratory markers of disease severity have to be present, four major co-morbidities (pneumonia, meningitis, bacteraemia or gastroenteritis with severe dehydration) are excluded, and a Plasmodium falciparum parasite density threshold is introduced, in order to maximize the specificity of the case definition. Secondary case definitions allow inclusion of co-morbidities and/or allow for the presence of parasitaemia at any density. The programmatic implementation of standardized case assessment included a clinical algorithm for evaluating seriously sick children, improvements to care delivery and a robust training and evaluation programme for clinicians.
Conclusions: The case definition developed for the pivotal phase III RTS, S vaccine study is consistent with WHO recommendations, is locally applicable and appropriately balances sensitivity and specificity in the diagnosis of severe malaria. Processes set up to standardize severe malaria data collection will allow robust assessment of the efficacy of the RTS, S vaccine against severe malaria, strengthen local capacity and benefit patient care for subjects in the trial.
Trial registration: Clinicaltrials.gov NCT00866619.
Figures


References
-
- WHO. World malaria report 2009. World Health Organization (WHO); 2009. ISBN 978 92 4 156390 1.
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitié MA, Dubovsky F, Menéndez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. - DOI - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, Sigauque B, Milman J, Mandomando I, Bassat Q, Guinovart C, Espasa M, Corachan S, Lievens M, Navia MM, Dubois MC, Menendez C, Dubovsky F, Cohen J, Thompson R, Ballou WR. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. - DOI - PubMed
-
- Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, Demoitié MA, Stallaert JF, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, von Seidlein L. Efficacy of RTS, S/AS01 vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

