Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy
- PMID: 21816228
- PMCID: PMC3188316
- DOI: 10.1016/j.joca.2011.07.005
Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy
Abstract
Objective: This study investigated a novel approach to induce chondrogenic differentiation of human mesenchymal stem cells (hMSC). We hypothesized that a structured three-dimensional co-culture using hMSC and chondrocytes would provide chondroinductive cues to hMSC without inducing hypertrophy.
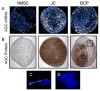
Method: In an effort to promote optimal chondrogenic differentiation of hMSC, we created bilaminar cell pellets (BCPs), which consist of a spherical population of hMSC encased within a layer of juvenile chondrocytes (JC). In addition to histologic analyses, we examined proteoglycan content and expression of chondrogenic and hypertrophic genes in BCPs, JC pellets, and hMSC pellets grown in the presence or absence of transforming growth factor-β (TGFβ) following 21 days of culture in either growth or chondrogenic media.
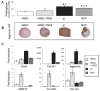
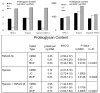
Results: In either growth or chondrogenic media, we observed that BCPs and JC pellets produced more proteoglycan than hMSC pellets treated with TGFβ. BCPs and JC pellets also exhibited higher expression of the chondrogenic genes Sox9, aggrecan, and collagen 2A1, and lower expression of the hypertrophic genes matrix metalloproteinase-13, Runx2, collagen 1A1, and collagen 10A1 than hMSC pellets. Histologic analyses suggest that JC promote chondrogenic differentiation of cells in BCPs without hypertrophy. Furthermore, when cultured in hypoxic and inflammatory conditions intended to mimic the injured joint microenvironment, BCPs produced significantly more proteoglycan than either JC pellets or hMSC pellets.
Conclusion: The BCP co-culture promotes a chondrogenic phenotype without hypertrophy and, relative to pellet cultures of hMSCs or JCs alone, is more resistant to the adverse conditions anticipated at the site of articular cartilage repair.
Copyright © 2011 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



References
-
- Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. - PubMed
-
- Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39 1:S58–65. - PubMed
-
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

