Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons
- PMID: 21816272
- PMCID: PMC3151530
- DOI: 10.1016/j.cell.2011.07.007
Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons
Retraction in
-
Retraction notice to: Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons.Cell. 2014 Apr 10;157(2):514. doi: 10.1016/j.cell.2014.03.002. Cell. 2014. PMID: 24843893 Free PMC article. No abstract available.
Abstract
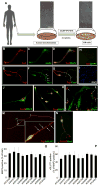
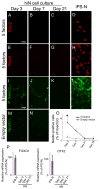
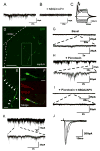
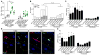
Directed conversion of mature human cells, as from fibroblasts to neurons, is of potential clinical utility for neurological disease modeling as well as cell therapeutics. Here, we describe the efficient generation of human-induced neuronal (hiN) cells from adult skin fibroblasts of unaffected individuals and Alzheimer's patients, using virally transduced transcription regulators and extrinsic support factors. hiN cells from unaffected individuals display morphological, electrophysiological, and gene expression profiles that typify glutamatergic forebrain neurons and are competent to integrate functionally into the rodent CNS. hiN cells from familial Alzheimer disease (FAD) patients with presenilin-1 or -2 mutations exhibit altered processing and localization of amyloid precursor protein (APP) and increased production of Aβ, relative to the source patient fibroblasts or hiN cells from unaffected individuals. Together, our findings demonstrate directed conversion of human fibroblasts to a neuronal phenotype and reveal cell type-selective pathology in hiN cells derived from FAD patients.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Comment in
- Regen Med. 2011 Nov;6(6):685-7
-
Cell intrinsic pathology in human fibroblast-derived neurons from Alzheimer's disease patients.Regen Med. 2012 Jan;7(1):25-8. doi: 10.2217/rme.11.102. Regen Med. 2012. PMID: 22168493 No abstract available.
-
Findings of research misconduct.NIH Guide Grants Contracts (Bethesda). 2015 Apr 17:NOT-OD-15-092. NIH Guide Grants Contracts (Bethesda). 2015. PMID: 25898420 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2015 Apr 7;80(66):18638-18639. Fed Regist. 2015. PMID: 27768265 Free PMC article. No abstract available.
References
-
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat Psych-Gerichtl Med. 1907;64:146–148.
-
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

