Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis
- PMID: 21816733
- PMCID: PMC3191560
- DOI: 10.1136/bmj.d4588
Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis
Abstract
Objective: To evaluate the accumulated information from genetic association studies investigating the impact of variants of the cytochrome P450 (CYP) 2C19 genotype on the clinical efficacy of clopidogrel.
Design: Systematic review and meta-analysis with a structured search algorithm and prespecified eligibility criteria for retrieval of relevant studies; dominant genetic model assumptions and quantitative methods for calculating summary effect estimates from study level odds ratios; systematic assessment of bias within and between studies; and grading of the cumulative evidence by consensus criteria.
Data sources: Medline, Embase, the Cochrane Library, online databases, contents pages and bibliographies of general medical, cardiovascular, pharmacological, and genetic journals. Eligibility criteria for selecting studies Original full length reports assessing the cumulative incidence of major adverse cardiovascular events or stent thrombosis over a follow-up period of at least a month in association with carrier status for the loss of function or gain of function CYP2C19 allele in adult patients with coronary artery disease and a clinical presentation of acute coronary syndrome or stable angina pectoris who were taking clopidogrel.
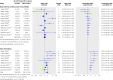
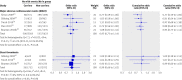
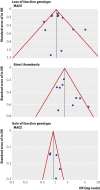
Results: 15 studies met the inclusion criteria. The random effects summary odds ratio for stent thrombosis in carriers of at least one CYP2C19 loss of function allele versus non-carriers combining nine studies was 1.77 (95% confidence interval 1.31 to 2.40; P < 0.001). This nominally significant odds ratio was subject to considerable bias across the studies (small study effect bias and replication diversity). The adjustment for these quality modifiers tended to abolish the association. The corresponding random effects summary odds ratio of major adverse cardiovascular events for 12 studies combined was 1.11 (0.89 to 1.39; P = 0.36). The random effects summary odds ratio of stent thrombosis in carriers versus non-carriers of at least one CYP2C19*17 gain of function allele for three studies combined was 0.99 (0.60 to 1.62; P = 0.96), and the corresponding odds ratio of major adverse cardiovascular events in five studies was 0.93 (0.75 to 1.14; P = 0.48). The overall quality of epidemiological evidence was graded as low, which excludes reliable clinical assessments.
Conclusions: Accumulated information from genetic association studies does not indicate a substantial or consistent influence of CYP2C19 gene polymorphisms on the clinical efficacy of clopidogrel. The current evidence does not support the use of individualised antiplatelet regimens guided by CYP2C19 genotype.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Putting genomics into practice.BMJ. 2011 Aug 4;343:d4953. doi: 10.1136/bmj.d4953. BMJ. 2011. PMID: 21816737 No abstract available.
-
ACP Journal Club. Review: Reduced function CYP2C19 genotypes may increase risk for stent clots in patients receiving clopidogrel.Ann Intern Med. 2011 Dec 20;155(12):JC6-13. doi: 10.7326/0003-4819-155-12-201112200-02013. Ann Intern Med. 2011. PMID: 22184714 No abstract available.
References
-
- King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 writing group to review new evidence and update the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention, writing on behalf of the 2005 writing committee. Circulation 2008;117:261-95. - PubMed
-
- Debnath B, Al-Mawsawi LQ, Neamati N. Are we living in the end of the blockbuster drug era? Drug News Perspect 2010;23:670-84. - PubMed
-
- Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J 2007;154:221-31. - PubMed
-
- Sofi F, Marcucci R, Gori AM, Giusti B, Abbate R, Gensini GF. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysis. Thromb Haemost 2010;103:841-8. - PubMed
-
- Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919-33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
