Biochemical impact of the host adaptation-associated PB2 E627K mutation on the temperature-dependent RNA synthesis kinetics of influenza A virus polymerase complex
- PMID: 21816827
- PMCID: PMC3186381
- DOI: 10.1074/jbc.M111.262048
Biochemical impact of the host adaptation-associated PB2 E627K mutation on the temperature-dependent RNA synthesis kinetics of influenza A virus polymerase complex
Abstract
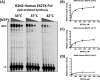
Most avian influenza A viruses, which preferentially replicate at the high temperatures found in the digestive tract of birds, have a glutamic acid at residue 627 of the viral RNA polymerase PB2 subunit (Glu-627), whereas the human viruses, which optimally replicate at the low temperatures observed in the human respiratory tract, have a lysine (Lys-627). The mechanism of action for this mutation is still not understood, although interaction with host factors has been proposed to play a major role. In this study, we explored an alternative, yet related, hypothesis that this PB2 mutation may alter the temperature-dependent enzymatic polymerase activity of the viral polymerase. First, the avian polymerase protein, which was purified from baculovirus expression system, indeed remained significantly active at higher temperatures (i.e. 37 and 42 °C), whereas the human E627K mutant drastically lost activity at these high temperatures. Second, our steady-state kinetics data revealed that the human E627K mutant polymerase is catalytically more active than the avian Glu-627 polymerase at 34 °C. Importantly, the E627K mutation elevates apparent K(cat) at low temperatures with little effect on K(m), suggesting that the E627K mutation alters the biochemical steps involved in enzyme catalysis rather than the interaction with the incoming NTP. Third, this temperature-dependent kinetic impact of the human E627K mutation was also observed with different RNA templates, with different primers and also in the presence of nucleoprotein. In conclusion, our study suggests that the amino acid sequence variations at residue 627 of PB2 subunit can directly alter the enzyme kinetics of influenza polymerase.
Figures


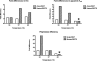

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

