Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency
- PMID: 21816832
- PMCID: PMC3186343
- DOI: 10.1182/blood-2011-06-365049
Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency
Abstract
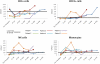
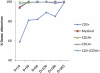
We performed nonmyeloablative HSCT in 6 patients with a newly described genetic immunodeficiency syndrome caused by mutations in GATA2-a disease characterized by nontuberculous mycobacterial infection, monocytopenia, B- and NK-cell deficiency, and the propensity to transform to myelodysplastic syndrome/acute myelogenous leukemia. Two patients received peripheral blood stem cells (PBSCs) from matched-related donors, 2 received PBSCs from matched-unrelated donors, and 2 received stem cells from umbilical cord blood (UCB) donors. Recipients of matched-related and -unrelated donors received fludarabine and 200 cGy of total body irradiation (TBI); UCB recipients received cyclophosphamide in addition to fludarabine and TBI as conditioning. All patients received tacrolimus and sirolimus posttransplantation. Five patients were alive at a median follow-up of 17.4 months (range, 10-25). All patients achieved high levels of donor engraftment in the hematopoietic compartments that were deficient pretransplantation. Adverse events consisted of delayed engraftment in the recipient of a single UCB, GVHD in 4 patients, and immune-mediated pancytopenia and nephrotic syndrome in the recipient of a double UCB transplantation. Nonmyeloablative HSCT in GATA2 deficiency results in reconstitution of the severely deficient monocyte, B-cell, and NK-cell populations and reversal of the clinical phenotype. Registered at www.clinicaltrials.gov as NCT00923364.
Figures
References
-
- Scott HS, Hahn CN, Carmichael CL, et al. GATA2 is a new predisposition gene for familial myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) [abstract]. Blood (ASH Annual Meeting Abstracts) 2010;116(21):Abstract LBA–3.