Estrogen sulfotransferase inhibits adipocyte differentiation
- PMID: 21816900
- PMCID: PMC3165918
- DOI: 10.1210/me.2011-1089
Estrogen sulfotransferase inhibits adipocyte differentiation
Abstract
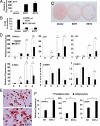
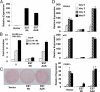
The estrogen sulfotransferase (EST) is a phase II drug-metabolizing enzyme known to catalyze the sulfoconjugation of estrogens. EST is highly expressed in the white adipose tissue of male mice, but the role of EST in the development and function of adipocytes remains largely unknown. In this report, we showed that EST played an important role in adipocyte differentiation. EST was highly expressed in 3T3-L1 preadipocytes and primary mouse preadipocytes. The expression of EST was dramatically reduced in differentiated 3T3-L1 cells and mature primary adipocytes. Overexpression of EST in 3T3-L1 cells prevented adipocyte differentiation. In contrast, preadipocytes isolated from EST knockout (EST-/-) mice exhibited enhanced differentiation. The inhibitory effect of EST on adipogenesis likely resulted from the sustained activation of ERK1/2 MAPK and inhibition of insulin signaling, leading to a failure of switch from clonal expansion to differentiation. The enzymatic activity of EST was required for the inhibitory effect of EST on adipogenesis, because an enzyme-dead EST mutant failed to inhibit adipocyte differentiation. In vivo, overexpression of EST in the adipose tissue of female transgenic mice resulted in smaller adipocyte size. Taken together, our results suggest that EST functions as a negative regulator of adipogenesis.
Figures




Similar articles
-
Inhibition of MMP-13 prevents diet-induced obesity in mice and suppresses adipogenesis in 3T3-L1 preadipocytes.Mol Biol Rep. 2015 Jul;42(7):1225-32. doi: 10.1007/s11033-015-3861-2. Epub 2015 Feb 15. Mol Biol Rep. 2015. PMID: 25682268
-
Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1 preadipocytes.Am J Physiol Endocrinol Metab. 2009 Dec;297(6):E1378-87. doi: 10.1152/ajpendo.00252.2009. Epub 2009 Oct 6. Am J Physiol Endocrinol Metab. 2009. PMID: 19808909 Free PMC article.
-
The chalcones cardamonin and flavokawain B inhibit the differentiation of preadipocytes to adipocytes by activating ERK.Arch Biochem Biophys. 2014 Jul 15;554:44-54. doi: 10.1016/j.abb.2014.05.008. Epub 2014 May 17. Arch Biochem Biophys. 2014. PMID: 24845100
-
Estrogen sulfotransferase regulates body fat and glucose homeostasis in female mice.Am J Physiol Endocrinol Metab. 2010 Oct;299(4):E657-64. doi: 10.1152/ajpendo.00707.2009. Epub 2010 Aug 3. Am J Physiol Endocrinol Metab. 2010. PMID: 20682840 Free PMC article.
-
APPL1 knockdown blocks adipogenic differentiation and promotes adipocyte lipolysis.Mol Cell Endocrinol. 2020 Apr 15;506:110755. doi: 10.1016/j.mce.2020.110755. Epub 2020 Feb 8. Mol Cell Endocrinol. 2020. PMID: 32045627
Cited by
-
Estrogen sulfotransferase/SULT1E1 promotes human adipogenesis.Mol Cell Biol. 2014 May;34(9):1682-94. doi: 10.1128/MCB.01147-13. Epub 2014 Feb 24. Mol Cell Biol. 2014. PMID: 24567372 Free PMC article.
-
Complex roles for sulfation in the toxicities of polychlorinated biphenyls.Crit Rev Toxicol. 2024 Feb;54(2):92-122. doi: 10.1080/10408444.2024.2311270. Epub 2024 Feb 16. Crit Rev Toxicol. 2024. PMID: 38363552 Free PMC article. Review.
-
Estrogen Deficiency and the Origin of Obesity during Menopause.Biomed Res Int. 2014;2014:757461. doi: 10.1155/2014/757461. Epub 2014 Mar 6. Biomed Res Int. 2014. PMID: 24734243 Free PMC article.
-
SULT1E1 inhibits cell proliferation and invasion by activating PPARγ in breast cancer.J Cancer. 2018 Feb 28;9(6):1078-1087. doi: 10.7150/jca.23596. eCollection 2018. J Cancer. 2018. PMID: 29581787 Free PMC article.
-
WISP1/CCN4 inhibits adipocyte differentiation through repression of PPARγ activity.Sci Rep. 2017 May 11;7(1):1749. doi: 10.1038/s41598-017-01866-2. Sci Rep. 2017. PMID: 28496206 Free PMC article.
References
-
- Hu E, Kim JB, Sarraf P, Spiegelman BM. 1996. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 274:2100–2103 - PubMed
-
- MacDougald OA, Cornelius P, Liu R, Lane MD. 1995. Insulin regulates transcription of the CCAAT/enhancer binding protein (C/EBP) α, β, and δ genes in fully-differentiated 3T3–L1 adipocytes. J Biol Chem 270:647–654 - PubMed
-
- Tontonoz P, Hu E, Spiegelman BM. 1994. Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell 79:1147–1156 - PubMed
-
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. 1999. Cross-regulation of C/EBP α and PPAR γ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 3:151–158 - PubMed
-
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. 2000. Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

