Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence
- PMID: 21816908
- PMCID: PMC3185156
- DOI: 10.1158/0008-5472.CAN-11-1341
Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence
Abstract
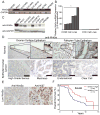
Epithelial ovarian cancer (EOC) remains the most lethal gynecologic malignancy in the United States. Thus, there is an urgent need to develop novel therapeutics for this disease. Cellular senescence is an important tumor suppression mechanism that has recently been suggested as a novel mechanism to target for developing cancer therapeutics. Wnt5a is a noncanonical Wnt ligand that plays a context-dependent role in human cancers. Here, we investigate the role of Wnt5a in regulating senescence of EOC cells. We show that Wnt5a is expressed at significantly lower levels in human EOC cell lines and in primary human EOCs (n = 130) compared with either normal ovarian surface epithelium (n = 31; P = 0.039) or fallopian tube epithelium (n = 28; P < 0.001). Notably, a lower level of Wnt5a expression correlates with tumor stage (P = 0.003) and predicts shorter overall survival in EOC patients (P = 0.003). Significantly, restoration of Wnt5a expression inhibits the proliferation of human EOC cells both in vitro and in vivo in an orthotopic EOC mouse model. Mechanistically, Wnt5a antagonizes canonical Wnt/β-catenin signaling and induces cellular senescence by activating the histone repressor A/promyelocytic leukemia senescence pathway. In summary, we show that loss of Wnt5a predicts poor outcome in EOC patients and Wnt5a suppresses the growth of EOC cells by triggering cellular senescence. We suggest that strategies to drive senescence in EOC cells by reconstituting Wnt5a signaling may offer an effective new strategy for EOC therapy.
Figures





Similar articles
-
Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer.Cell Cycle. 2014;13(2):199-207. doi: 10.4161/cc.26953. Epub 2013 Oct 29. Cell Cycle. 2014. PMID: 24200970 Free PMC article.
-
Lysophosphatidic Acid Initiates Epithelial to Mesenchymal Transition and Induces β-Catenin-mediated Transcription in Epithelial Ovarian Carcinoma.J Biol Chem. 2015 Sep 4;290(36):22143-54. doi: 10.1074/jbc.M115.641092. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175151 Free PMC article.
-
Wnt5a influences viability, migration, adhesion, colony formation, E- and N-cadherin expression of human ovarian cancer cell line SKOV-3.Folia Biol (Praha). 2014;60(2):57-67. doi: 10.14712/fb2014060020057. Folia Biol (Praha). 2014. PMID: 24785108
-
Wnt5a: A promising therapeutic target in ovarian cancer.Pathol Res Pract. 2021 Mar;219:153348. doi: 10.1016/j.prp.2021.153348. Epub 2021 Jan 27. Pathol Res Pract. 2021. PMID: 33540373 Review.
-
Wnt-signaling and senescence: A tug of war in early neoplasia?Cancer Biol Ther. 2008 Nov;7(11):1706-11. doi: 10.4161/cbt.7.11.6943. Epub 2008 Nov 7. Cancer Biol Ther. 2008. PMID: 18836285 Free PMC article. Review.
Cited by
-
Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis.Cancer Res. 2013 Jun 15;73(12):3649-60. doi: 10.1158/0008-5472.CAN-12-3832. Cancer Res. 2013. PMID: 23771907 Free PMC article.
-
WNT signalling pathways as therapeutic targets in cancer.Nat Rev Cancer. 2013 Jan;13(1):11-26. doi: 10.1038/nrc3419. Nat Rev Cancer. 2013. PMID: 23258168 Review.
-
Biological functions of macrophage-derived Wnt5a, and its roles in human diseases.Oncotarget. 2016 Oct 11;7(41):67674-67684. doi: 10.18632/oncotarget.11874. Oncotarget. 2016. PMID: 27608847 Free PMC article. Review.
-
Loss of Claudin-4 Reduces DNA Damage Repair and Increases Sensitivity to PARP Inhibitors.Mol Cancer Ther. 2022 Apr 1;21(4):647-657. doi: 10.1158/1535-7163.MCT-21-0827. Mol Cancer Ther. 2022. PMID: 35373300 Free PMC article.
-
CARM1-expressing ovarian cancer depends on the histone methyltransferase EZH2 activity.Nat Commun. 2018 Feb 12;9(1):631. doi: 10.1038/s41467-018-03031-3. Nat Commun. 2018. PMID: 29434212 Free PMC article.
References
-
- Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell. 2009;36:2–14. - PubMed
-
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

