Identification of inhibitors against Mycobacterium tuberculosis thiamin phosphate synthase, an important target for the development of anti-TB drugs
- PMID: 21818324
- PMCID: PMC3144219
- DOI: 10.1371/journal.pone.0022441
Identification of inhibitors against Mycobacterium tuberculosis thiamin phosphate synthase, an important target for the development of anti-TB drugs
Abstract
Tuberculosis (TB) continues to pose a serious challenge to human health afflicting a large number of people throughout the world. In spite of the availability of drugs for the treatment of TB, the non-compliance to 6-9 months long chemotherapeutic regimens often results in the emergence of multidrug resistant strains of Mycobacterium tuberculosis adding to the precariousness of the situation. This has necessitated the development of more effective drugs. Thiamin biosynthesis, an important metabolic pathway of M. tuberculosis, is shown to be essential for the intracellular growth of this pathogen and hence, it is believed that inhibition of this pathway would severely affect the growth of M. tuberculosis. In this study, a comparative homology model of M. tuberculosis thiamin phosphate synthase (MtTPS) was generated and employed for virtual screening of NCI diversity set II to select potential inhibitors. The best 39 compounds based on the docking results were evaluated for their potential to inhibit the MtTPS activity. Seven compounds inhibited MtTPS activity with IC(50) values ranging from 20-100 µg/ml and two of these exhibited weak inhibition of M. tuberculosis growth with MIC(99) values being 125 µg/ml and 162.5 µg/ml while one compound was identified as a very potent inhibitor of M. tuberculosis growth with an MIC(99) value of 6 µg/ml. This study establishes MtTPS as a novel drug target against M. tuberculosis leading to the identification of new lead molecules for the development of antitubercular drugs. Further optimization of these lead compounds could result in more potent therapeutic molecules against Tuberculosis.
Conflict of interest statement
Figures

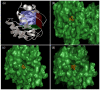
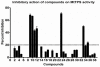


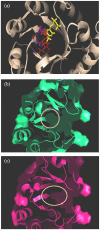

Similar articles
-
Screening of antitubercular compound library identifies novel ATP synthase inhibitors of Mycobacterium tuberculosis.Tuberculosis (Edinb). 2018 Jan;108:56-63. doi: 10.1016/j.tube.2017.10.008. Epub 2017 Oct 25. Tuberculosis (Edinb). 2018. PMID: 29523328
-
Development of potent chemical antituberculosis agents targeting Mycobacterium tuberculosis acetohydroxyacid synthase.Int J Antimicrob Agents. 2016 Sep;48(3):247-58. doi: 10.1016/j.ijantimicag.2016.04.031. Epub 2016 Jul 6. Int J Antimicrob Agents. 2016. PMID: 27451857
-
Exploring the Potential Inhibition of Candidate Drug Molecules for Clinical Investigation Based on their Docking or Crystallographic Analyses against M. tuberculosis Enzyme Targets.Curr Top Med Chem. 2020;20(29):2662-2680. doi: 10.2174/1568026620666200903163921. Curr Top Med Chem. 2020. PMID: 32885754 Review.
-
A novel inhibitor of indole-3-glycerol phosphate synthase with activity against multidrug-resistant Mycobacterium tuberculosis.FEBS J. 2009 Jan;276(1):144-54. doi: 10.1111/j.1742-4658.2008.06763.x. Epub 2008 Nov 20. FEBS J. 2009. PMID: 19032598
-
Mycobacterial tuberculosis Enzyme Targets and their Inhibitors.Curr Top Med Chem. 2019;19(5):337-355. doi: 10.2174/1568026619666190219105722. Curr Top Med Chem. 2019. PMID: 30806318 Review.
Cited by
-
Transposon sequencing reveals metabolic pathways essential for Mycobacterium tuberculosis infection.PLoS Pathog. 2024 Mar 18;20(3):e1011663. doi: 10.1371/journal.ppat.1011663. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38498580 Free PMC article.
-
Functional Analysis of Hypothetical Proteins of Vibrio parahaemolyticus Reveals the Presence of Virulence Factors and Growth-Related Enzymes With Therapeutic Potential.Bioinform Biol Insights. 2022 Nov 9;16:11779322221136002. doi: 10.1177/11779322221136002. eCollection 2022. Bioinform Biol Insights. 2022. PMID: 36386863 Free PMC article.
-
Potential Role of Vitamins A, B, C, D and E in TB Treatment and Prevention: A Narrative Review.Antibiotics (Basel). 2021 Nov 5;10(11):1354. doi: 10.3390/antibiotics10111354. Antibiotics (Basel). 2021. PMID: 34827292 Free PMC article. Review.
-
Molecular Architect: A User-Friendly Workflow for Virtual Screening.ACS Omega. 2020 Mar 20;5(12):6628-6640. doi: 10.1021/acsomega.9b04403. eCollection 2020 Mar 31. ACS Omega. 2020. PMID: 32258898 Free PMC article.
-
The ThiL enzyme is a valid antibacterial target essential for both thiamine biosynthesis and salvage pathways in Pseudomonas aeruginosa.J Biol Chem. 2020 Jul 17;295(29):10081-10091. doi: 10.1074/jbc.RA120.013295. Epub 2020 May 13. J Biol Chem. 2020. PMID: 32404369 Free PMC article.
References
-
- Breslow R. On the mechanism of thiamin action. IV. Evidence from studies on model systems. J Am Chem Soc. 1958;80:3719–3726.
-
- Bettendorff L, Wins P. Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 2009;276:2917–2925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

