Herpes simplex virus-induced epithelial damage and susceptibility to human immunodeficiency virus type 1 infection in human cervical organ culture
- PMID: 21818356
- PMCID: PMC3144918
- DOI: 10.1371/journal.pone.0022638
Herpes simplex virus-induced epithelial damage and susceptibility to human immunodeficiency virus type 1 infection in human cervical organ culture
Abstract
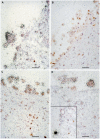
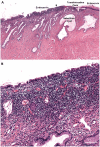
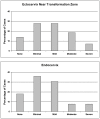
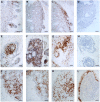
Normal human premenopausal cervical tissue has been used to derive primary cell populations and to establish ex vivo organ culture systems to study infections with herpes simplex virus (HSV-1 or HSV-2) and human immunodeficiency virus type 1 (HIV-1). Infection with either HSV-1 or HSV-2 rapidly induced multinuclear giant cell formation and widespread damage in mucosal epithelial cells. Subsequent exposure of the damaged mucosal surfaces to HIV-1 revealed frequent co-localization of HSV and HIV-1 antigens. The short-term organ culture system provides direct experimental support for the epidemiological findings that pre-existing sexually transmitted infections, including primary and recurrent herpes virus infections at mucosal surfaces, represent major risk factors for acquisition of primary HIV-1 infection. Epithelial damage in combination with pre-existing inflammation, as described here for overtly normal human premenopausal cervix, creates a highly susceptible environment for the initiation and establishment of primary HIV-1 infection in the sub-mucosa of the cervical transformation zone.
Conflict of interest statement
Figures



References
-
- Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. - PubMed
-
- Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. Aids. 2003;17:455–480. - PubMed
-
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–852. - PubMed
-
- Johnston MI, Fauci AS. An HIV vaccine–evolving concepts. N Engl J Med. 2007;356:2073–2081. - PubMed
-
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. - PubMed

