Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis
- PMID: 21818404
- PMCID: PMC3144950
- DOI: 10.1371/journal.pone.0022887
Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis
Abstract
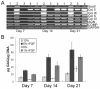
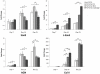
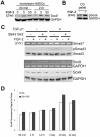
Human mesenchymal stem cells (hMSCs) are multipotent cells capable of differentiating into a variety of mature cell types, including osteoblasts, adipocytes and chondrocytes. It has previously been shown that, when expanded in medium supplemented with fibroblast growth factor-2 (FGF-2), hMSCs show enhanced chondrogenesis (CG). Previous work concluded that the enhancement of CG could be attributed to the selection of a cell subpopulation with inherent chondrogenic potential. In this study, we show that FGF-2 pretreatment actually primed hMSCs to undergo enhanced CG by increasing basal Sox9 protein levels. Our results show that Sox9 protein levels were elevated within 30 minutes of exposure to FGF-2 and progressively increased with longer exposures. Further, we show using flow cytometry that FGF-2 increased Sox9 protein levels per cell in proliferating and non-proliferating hMSCs, strongly suggesting that FGF-2 primes hMSCs for subsequent CG by regulating Sox9. Indeed, when hMSCs were exposed to FGF-2 for 2 hours and subsequently differentiated into the chondrogenic lineage using pellet culture, phosphorylated-Sox9 (pSox9) protein levels became elevated and ultimately resulted in an enhancement of CG. However, small interfering RNA (siRNA)-mediated knockdown of Sox9 during hMSC expansion was unable to negate the prochondrogenic effects of FGF-2, suggesting that the FGF-2-mediated enhancement of hMSC CG is only partly regulated through Sox9. Our findings provide new insights into the mechanism by which FGF-2 regulates predifferentiation hMSCs to undergo enhanced CG.
Conflict of interest statement
Figures

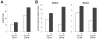
Similar articles
-
Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells.Tissue Eng Part A. 2010 Mar;16(3):1009-19. doi: 10.1089/ten.TEA.2009.0100. Tissue Eng Part A. 2010. PMID: 19842915 Free PMC article.
-
microRNA-495 inhibits chondrogenic differentiation in human mesenchymal stem cells by targeting Sox9.Stem Cells Dev. 2014 Aug 1;23(15):1798-808. doi: 10.1089/scd.2013.0609. Epub 2014 Apr 28. Stem Cells Dev. 2014. PMID: 24654627 Free PMC article.
-
FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells.J Cell Physiol. 2005 May;203(2):398-409. doi: 10.1002/jcp.20238. J Cell Physiol. 2005. PMID: 15521064
-
Metabolic activities and chondrogenic differentiation of human mesenchymal stem cells following recombinant adeno-associated virus-mediated gene transfer and overexpression of fibroblast growth factor 2.Tissue Eng Part A. 2011 Aug;17(15-16):1921-33. doi: 10.1089/ten.TEA.2011.0018. Epub 2011 May 4. Tissue Eng Part A. 2011. PMID: 21417714
-
Human Mesenchymal Stem Cells Pretreated with Interleukin-1β and Stimulated with Bone Morphogenetic Growth Factor-3 Enhance Chondrogenesis.Tissue Eng Part A. 2018 May;24(9-10):775-785. doi: 10.1089/ten.TEA.2017.0087. Epub 2017 Nov 30. Tissue Eng Part A. 2018. PMID: 28978269
Cited by
-
Stem cell-based tissue engineering approaches for musculoskeletal regeneration.Curr Pharm Des. 2013;19(19):3429-45. doi: 10.2174/13816128113199990350. Curr Pharm Des. 2013. PMID: 23432679 Free PMC article. Review.
-
Environmental preconditioning rejuvenates adult stem cells' proliferation and chondrogenic potential.Biomaterials. 2017 Feb;117:10-23. doi: 10.1016/j.biomaterials.2016.11.049. Epub 2016 Nov 25. Biomaterials. 2017. PMID: 27923196 Free PMC article. Review.
-
Reprogramming of Dermal Fibroblasts into Osteo-Chondrogenic Cells with Elevated Osteogenic Potency by Defined Transcription Factors.Stem Cell Reports. 2017 Jun 6;8(6):1587-1599. doi: 10.1016/j.stemcr.2017.04.018. Epub 2017 May 18. Stem Cell Reports. 2017. PMID: 28528696 Free PMC article.
-
Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy.Int J Mol Sci. 2018 Feb 10;19(2):537. doi: 10.3390/ijms19020537. Int J Mol Sci. 2018. PMID: 29439436 Free PMC article.
-
Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling.PLoS One. 2014 May 9;9(5):e96615. doi: 10.1371/journal.pone.0096615. eCollection 2014. PLoS One. 2014. PMID: 24816923 Free PMC article.
References
-
- Peat G, Wood L, Wilkie R, Thomas E, Grp KSS. How reliable is structured clinical history-taking in older adults with knee problems? Inter- and intraobserver variability of the KNE-SCI. Journal of Clinical Epidemiology. 2003;56:1030–1037. - PubMed
-
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. - PubMed
-
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. - PubMed
-
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. - PubMed
-
- Delorme B, Ringe J, Pontikoglou C, Gaillard J, Langonne A, et al. Specific lineage-priming of bone marrow mesenchymal stem cells provides the molecular framework for their plasticity. Stem Cells. 2009;27:1142–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

