Misfolded proteins in Alzheimer's disease and type II diabetes
- PMID: 21818468
- PMCID: PMC3210870
- DOI: 10.1039/c1cs15112f
Misfolded proteins in Alzheimer's disease and type II diabetes
Abstract
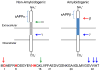
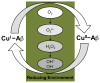
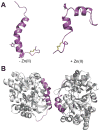
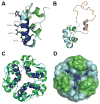
This tutorial review presents descriptions of two amyloidogenic proteins, amyloid-β (Aβ) peptides and islet amyloid polypeptide (IAPP), whose misfolding propensities are implicated in Alzheimer's disease (AD) and type II diabetes, respectively. Protein misfolding diseases share similarities, as well as some unique protein-specific traits, that could contribute to the initiation and/or development of their associated conditions. Aβ and IAPP are representative amyloidoses and are used to highlight some of the primary considerations for studying misfolded proteins associated with human diseases in this review. Among these factors, their physiological formation, aggregation, interactions with metal ions and other protein partners, and toxicity are presented. Small molecules that target and modulate the metal-Aβ interaction and neurotoxicity are included to illustrate one of the current approaches for uncovering the complexities of protein misfolding at the molecular level.
Figures


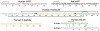

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

