Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum
- PMID: 21818701
- PMCID: PMC4334392
- DOI: 10.1007/s00281-011-0286-4
Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum
Abstract
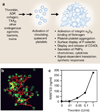
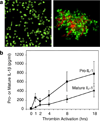
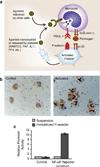
Platelets are chief effector cells in hemostasis. In addition, however, their specializations include activities and intercellular interactions that make them key effectors in inflammation and in the continuum of innate and adaptive immunity. This review focuses on the immune features of human platelets and platelets from experimental animals and on interactions between inflammatory, immune, and hemostatic activities of these anucleate but complex and versatile cells. The experimental findings and evidence for physiologic immune functions include previously unrecognized biologic characteristics of platelets and are paralleled by new evidence for unique roles of platelets in inflammatory, immune, and thrombotic diseases.
Figures






References
-
- Coller BS. A brief history of ideas about platelets in health and disease. In: Alan D, Michelson MD, editors. Platelets. 2nd edn. London: Elsevier; 2007. pp. xxiii–xlii.
-
- Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–1905. - PubMed
-
- Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. - PubMed
Publication types
MeSH terms
Grants and funding
- ULI-RR025764/RR/NCRR NIH HHS/United States
- HL044525/HL/NHLBI NIH HHS/United States
- R01 HL066277/HL/NHLBI NIH HHS/United States
- HL066277/HL/NHLBI NIH HHS/United States
- R01 HL091754/HL/NHLBI NIH HHS/United States
- K231440921/PHS HHS/United States
- HL091754/HL/NHLBI NIH HHS/United States
- R01 HL044525/HL/NHLBI NIH HHS/United States
- HL092746/HL/NHLBI NIH HHS/United States
- R01 HL048872/HL/NHLBI NIH HHS/United States
- R01 HL090870/HL/NHLBI NIH HHS/United States
- HL048872/HL/NHLBI NIH HHS/United States
- HL090870/HL/NHLBI NIH HHS/United States
- R37 HL044525/HL/NHLBI NIH HHS/United States
- C06 RR011234/RR/NCRR NIH HHS/United States
- R01 HL092746/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

