Building the posterior lateral line system in zebrafish
- PMID: 21818862
- PMCID: PMC3376715
- DOI: 10.1002/dneu.20962
Building the posterior lateral line system in zebrafish
Abstract
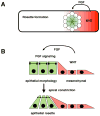
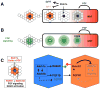
The posterior lateral line (pLL) in zebrafish has emerged as an excellent system to study how a sensory organ system develops. Here we review recent studies that illustrate how interactions between multiple signaling pathways coordinate cell fate,morphogenesis, and collective migration of cells in the posterior lateral line primordium. These studies also illustrate how the pLL system is contributing much more broadly to our understanding of mechanisms operating during the growth, regeneration, and self-organization of other organ systems during development and disease.
Figures







References
-
- Aman A, Nguyen M, Piotrowski T. Wnt/beta-catenin dependent cell proliferation underlies segmented lateral line morphogenesis. Dev Biol. 2011;349:470–482. - PubMed
-
- Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. - PubMed
-
- Baker PC, Schroeder TE. Cytoplasmic filaments and morphogenetic movement in the amphibian neural tube. Developmental Biology. 1967;15:432–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

