Biophysics of α-synuclein membrane interactions
- PMID: 21819966
- PMCID: PMC3249522
- DOI: 10.1016/j.bbamem.2011.07.032
Biophysics of α-synuclein membrane interactions
Abstract
Membrane proteins participate in nearly all cellular processes; however, because of experimental limitations, their characterization lags far behind that of soluble proteins. Peripheral membrane proteins are particularly challenging to study because of their inherent propensity to adopt multiple and/or transient conformations in solution and upon membrane association. In this review, we summarize useful biophysical techniques for the study of peripheral membrane proteins and their application in the characterization of the membrane interactions of the natively unfolded and Parkinson's disease (PD) related protein, α-synuclein (α-syn). We give particular focus to studies that have led to the current understanding of membrane-bound α-syn structure and the elucidation of specific membrane properties that affect α-syn-membrane binding. Finally, we discuss biophysical evidence supporting a key role for membranes and α-syn in PD pathogenesis. This article is part of a Special Issue entitled: Membrane protein structure and function.
Copyright © 2011. Published by Elsevier B.V.
Figures

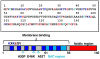
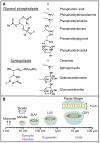
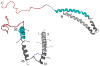
References
-
- Ahram M, Litou ZI, Fang R, Al-Tawallbeh G. Estimation of membrane proteins in the human proteome. In Silico Biol. 2006;6:379–386. - PubMed
-
- Doyle DA, Shipley GG. Membranes: Editorial overview. Curr Opin Struct Biol. 2009;19:369–371. - PubMed
-
- Lees A, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

